Figures & data
Figure 1. Experimental design to assess the efficacy of FUS and CD-40 combination against melanoma tumors. 0.5 × 106 B16F10 cells were injected subcutaneously (sc) in the right flank regions of C57/BL6 mice. 4 days later, the mice were injected with 0.125 × 106 cells in the left flank region by sc route. Unilateral treatment of the right flank tumor was initiated at a volume of 20–40 mm3. FUS heating (42–45°C) was applied for ∼15 min, and intratumoral injection of anti-CD-40 agonistic antibody (50 µg) was performed sequentially within 4 h of FUS heating. Red arrows indicate the three treatments with FUS and CD-40. Green arrow indicates the fourth anti-CD-40 dose. Mice were sacrificed when tumors reached >1 cm in any dimension or reached 30 days post-inoculation. The harvested treated tumor and spleen were analyzed for the population and type of immune cell.
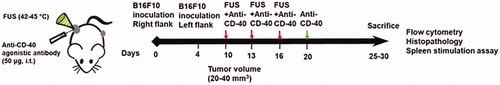
Figure 2. Local FUS therapy and in situ anti-CD-40 agonistic antibody suppressed the tumor growth of local and distant untreated site in B16F10 melanoma model. (a) Mean volumes of the treated tumors are shown till 30 days. Control and FUS reached sacrifice end points by day 21. CD-40 and FUS40 significantly decreased tumor volumes compared to FUS and untreated tumors; (b) Tumor weights at the time of sacrifice showed a significant reduction in the overall weight for FUS40 compared to other groups. (c) Representative images of the treated tumor. (d) Mean volumes of the distant untreated tumors are shown till 30 days. (e) Number of mice that were tumor free at the distant untreated site. Results are shown as mean ± SEM. One-way ANOVA followed by Fisher’s LSD without multiple comparisons correction. *p < .05, **p < .01.
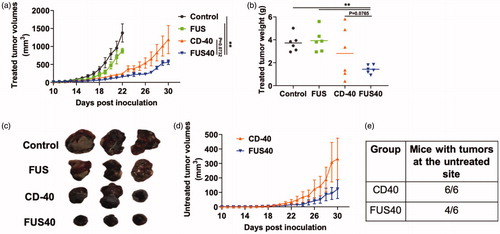
Figure 3. FUS40 enhanced the recruitment of leukocytes and prevented T-cell dysfunction. (a) Compared to other groups, FUS40-treated tumors exhibited relatively higher perivascular infiltration of lymphocytes (red box) within the tumor mass upon qualitative imaging by a veterinary pathologist blinded for the groups; n = 5, Hematoxylin:Eosin stain, Bar = 50 μm. (b) Enlarged view of FUS40 tumor sections (red box) showing perivascular infiltration of lymphocytes (black arrows), Bar = 20 μm. Differences were analyzed by an unpaired t test assuming unequal variance. (c) Flow cytometry showed that the frequency of tumor infiltrating leukocytes in FUS40 tumors was significantly greater than the control tumors. (d) Percentage of Granzyme-B + CD3+ CD8+ T cells was significantly higher for FUS40 (2–3-fold) compared to all other groups. FUS40 preserved activated CD8+ T cell from functional exhaustion by inhibiting PD-1 expression and enhancing Granzyme B production. For all channels, positive and negative cells were gated on the basis of fluorescence minus one control. Results are shown as mean ± SEM. *p < .05, Data were analyzed using a one-way ANOVA followed by Fisher’s LSD without multiple comparisons correction.
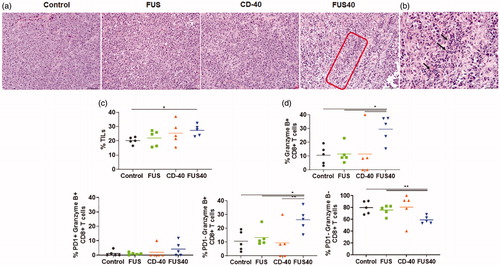
Figure 4. FUS40 revived the production of effector cytokines from melanoma specific CD4+ and CD8+ T cells in spleen. B16F10 melanoma bearing mice treated sequentially with FUS and anti-CD-40 agonistic antibody were sacrificed and spleen was evaluated for TRP-2 specific immunity in an ex vivo stimulation assay. (a) Flow cytometry contour plots representing the gating strategy for CD4+ and CD8+ T cells producing IL-2 and IFN-γ. (b) IL-2 and IFN-γ secreting CD4+ T cells in splenocytes after ex vivo TRP-2 stimulation were significantly increased by the FUS40 compared to control. Differences were analyzed by an unpaired t test assuming unequal variance. (c) The highest frequency of CD8+ T cells producing IL-2 and IFN-γ was observed in FUS40. *p < .05, **p < .01, one-way ANOVA followed by Fisher’s LSD without multiple comparisons correction.
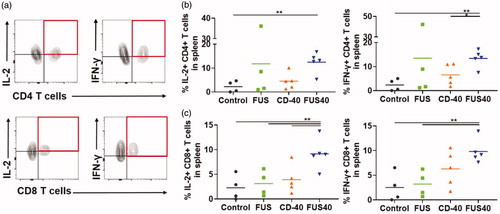
Figure 5. FUS40 promoted M1 macrophage polarization in the tumor and the spleen. (a) Frequency of M1 macrophages in the tumor was increased by 4-fold for FUS40 compared to FUS and control, whereas M2 macrophages in treated tumors remained unaltered compared to controls. CD11b + F4/80+ MHCII high (M1 macrophages) and CD11b + F4/80+ MHCII lo/neg CD206+ (M2 macrophages). (b) An increased percentage of M1 macrophages was observed in the spleens from CD-40 and FUS40 cohorts. FUS40 reduced the frequency of M2 macrophages in the spleen compared to other groups. Data are shown as mean ± SEM. Statistics were determined by ANOVA followed by Fisher’s LSD without multiple comparisons correction. *p < .05, **p < .01.
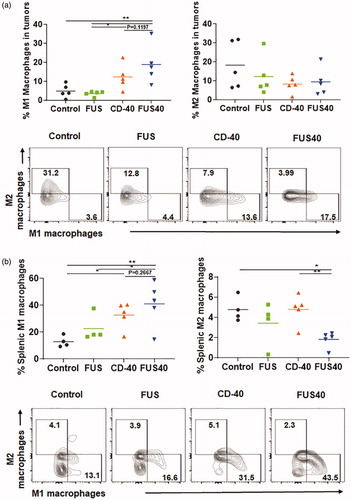
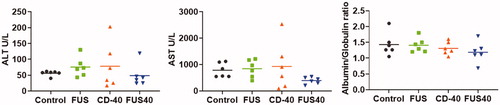
Figure 6. Local FUS40 and CD-40 therapy did not cause liver toxicity in B16F10 melanoma bearing mice. Levels of ALT, AST, and Albumin to Globulin ratio in the serum of mice were determined at the time of sacrifice 25–30 days post tumor inoculation. Data were analyzed by ANOVA followed by Fisher’s LSD without multiple comparisons correction (n = 6).