Figures & data
Table 1. Patients’ characteristics.
Table 2. Imaging features of tumors in hepatic dome.
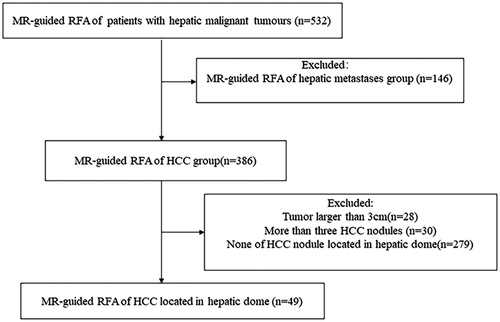
Figure 2. Recurrent HCC in the hepatic dome of a 59-year-old man treated with MR-guided RFA. (A) The recurrent nodule in segment VIII (arrow) is 15 mm in diameter and appears hyperintense in a coronal T2WI before RFA. (B–C) The RF electrode (arrow in B) is targeted gradually using the tilting of the puncture path under the coronal 3D-T1WI guidance. When the RF electrode reaches the edge of the nodule, the inner expandable multitined electrodes are expanded to 2.5 cm to overlap the nodule (arrow in C) without penetrating the diaphragm. (D–E) After RFA, the nodule is completely overlapped by the rim of hyperintensity on 3D-T1WI (arrow in D) and hypointensity on T2WI. Crescent-shaped effusions (arrow in E) are clearly displayed with the hyperintense signal in coronal T2WI between the liver capsule and the diaphragm. (F) The lesion (arrow) is completely ablated without a diaphragm injury at 3-month follow-up.
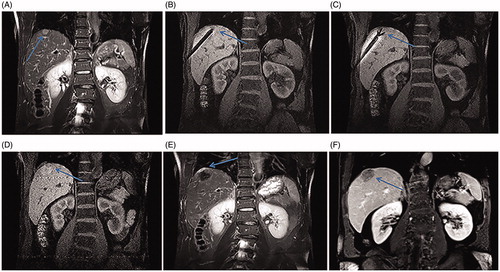
Figure 3. Recurrent HCC after liver resection in the hepatic dome of a 64-year-old man treated with MR-guided RFA. (A–D) The recurrent nodule, 12 mm in diameter, in the hepatic dome of segment II appears hyperintense in T2WI (arrow in A). Conventional TACE was carried out, and typical tumor staining is seen (arrow in B). At 8-month follow-up, the lesion is still growing up to 21 mm in diameter (arrow in C) with a rim of arterial phase hyper-enhancement (arrow in D) before RFA. (E–F) The RF electrode is targeted under the axial 3D-T1WI combination with sagittal oblique 3D-T1WI guidance. The inner expandable multitined electrodes are expanded to 3.0 cm to overlap the nodule (arrow in F) without penetrating the diaphragm and pericardium. (G) A typical ‘target sign’ (arrow) is clearly shown in the ablative zone with the hypointense nodule completely overlapped by the hyperintensity on 3D-T1WI, with a safe margin immediately after RFA. (H, I) The ablative zone with the ‘target sign’ (arrow) assimilated and without enhancement in MRI after 16-month follow-up.
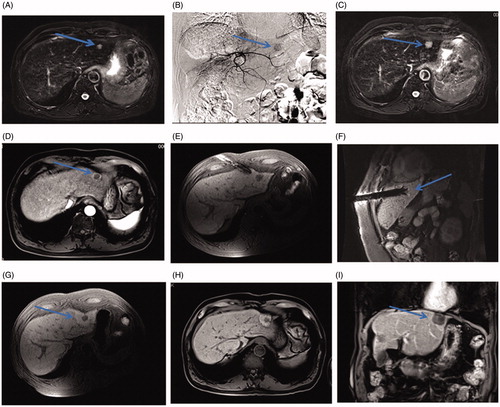
Figure 4. A small HCC in the hepatic dome of a 65-year-old woman with high serum AFP levels treated with MR-guided RFA using DWI. (A–D) The lesion in segment II, 13 mm in diameter, appears as an obvious enhancement in the arterial phase (arrow in C) and is hyperintense on the DWI (arrow, D) with isointensity on the T2WI and T1WI before RFA. (E–F) The RF electrode is targeted gradually using DWI-guidance (arrow in E) and clearly shows the placement of the expanded electrodes on the coronal 3D-T1WI. (G–I) Immediately after the RFA, the lesion is completely overlapped by the hyperintensity on 3D-T1WI and hypointensity on DWI (arrow in H) and T2WI (arrow in I).
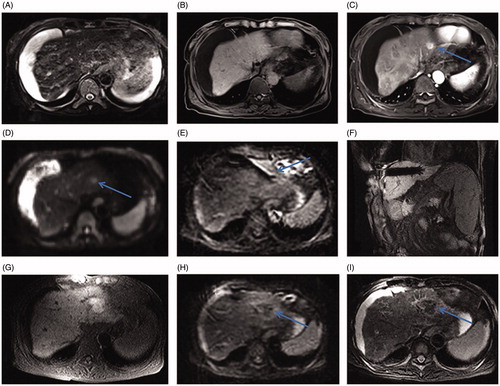
Figure 5. Small HCC in the hepatic dome of a 46-year-old man treated with MR-guided RFA. (A–B) The lesion in segment IV (arrow) is 21 mm in diameter and appears hypointense in T1WI (arrow, A) and hyperintense in T2WI (arrow, B) before RFA. (C) The RF multitined electrodes are expanded to overlap the lesion without penetrating the diaphragm and pericardium. (D–E) After the first RFA, the lesion is immediately overlapped partially by the rim of hyperintensity on 3D-T1WI and hypointensity on T2WI. The residual tumor of the left side still appears hypointense in T1WI (arrow in D) and hyperintense in T2WI (arrow in E) as pre-RFA. (F) A second ablation is performed after replacement of electrodes (arrow). (G, H) A typical ‘target sign’ (arrow in G) is clearly shown in the ablative zone on T1WI and appears hypointense in T2WI (arrow, H) immediately after RFA. (I) The ablative zone assimilated and without enhancement in MRI after 22-month follow-up.
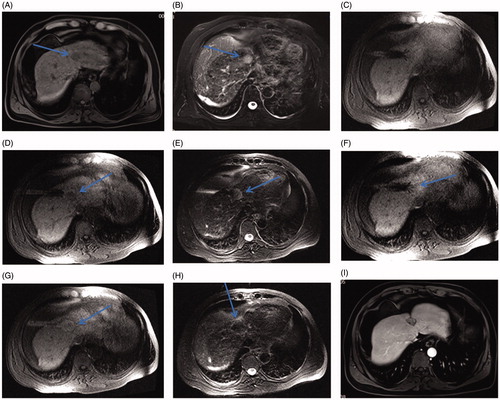
Table 3. Incidence of complications related to RFA.
Figure 6. Graph showing the probability of LPFS in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. The 1-, 3-, and 5-year LPFS rates are 98.0%.
![Figure 6. Graph showing the probability of LPFS in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. The 1-, 3-, and 5-year LPFS rates are 98.0%.](/cms/asset/c7f0292b-633f-4b24-be17-55f443a509b3/ihyt_a_1728397_f0006_c.jpg)
Figure 7. Probability of recurrence-free survival and estimated overall survival in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. (A) Graph showing overall recurrence-free survival. The estimated overall 1-, 3-, and 5-year recurrence-free survival rates are 68.1%, 39.9%, and 28.5%, respectively. (B) Graph showing overall survival estimation. The estimated overall 1-, 3-, and 5-year survival rates are 93.7%, 76.3%, and 54.3%, respectively.
![Figure 7. Probability of recurrence-free survival and estimated overall survival in 49 patients with 50 HCCs in the hepatic dome (mean diameter, 15.4 ± 5.8 mm [range, 7.0–29.0 mm]), treated with MR-guided RFA after a median follow-up of 31.0 months. (A) Graph showing overall recurrence-free survival. The estimated overall 1-, 3-, and 5-year recurrence-free survival rates are 68.1%, 39.9%, and 28.5%, respectively. (B) Graph showing overall survival estimation. The estimated overall 1-, 3-, and 5-year survival rates are 93.7%, 76.3%, and 54.3%, respectively.](/cms/asset/eeeddd4f-b175-4feb-b2c3-b664dc72440f/ihyt_a_1728397_f0007_c.jpg)