Figures & data
Figure 1. Workflow describing schematically all the methodological steps performed for advanced HTP. In HTP preparation, the patient anatomical model is obtained from CT segmentation. The vasculature, reconstructed from an MR scan and registered to the CT, is also included in this model. The dielectric properties are assigned to each tissue type based on the median DL-EPT values. The thermal properties are assigned from literature values, but an effective thermal conductivity accounting for convective heat transfer in the bladder is assigned. Next, treatment planning is performed, in which the electric field distribution is calculated and phase-amplitude settings for effective tumor heating are optimized. Finally, biological modeling predicts the radiosensitizing effect of hyperthermia in terms of an equivalent enhanced radiation dose.
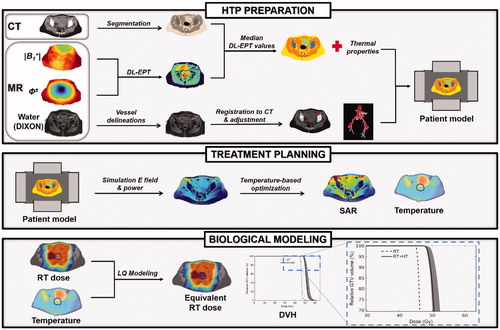
Table 1. Electrical and thermal properties of pelvic tissues at 70 MHz.
Table 2. Parameters and 95% confidence intervals (within brackets) used in the EQDRT calculation expressed in Equation (Equation5(5)
(5) ).
Figure 2. DL-EPT. Transverse and coronal cross-section of MR magnitude image from AFI sequence, |B1+| map, transceive phase (ϕ±) map, conductivity map obtained with DL-EPT (σDL). |B1+| and ϕ± maps are given as input to the trained network, which infers σDL. The tumor contour is shown in red. The tumor was delineated by a radiation oncologist on an ADC map (part of the diagnostic MRI protocol) [Citation85,Citation86]. The tumor delineation was then rigidly transferred to the frame of reference of the AFI image.
![Figure 2. DL-EPT. Transverse and coronal cross-section of MR magnitude image from AFI sequence, |B1+| map, transceive phase (ϕ±) map, conductivity map obtained with DL-EPT (σDL). |B1+| and ϕ± maps are given as input to the trained network, which infers σDL. The tumor contour is shown in red. The tumor was delineated by a radiation oncologist on an ADC map (part of the diagnostic MRI protocol) [Citation85,Citation86]. The tumor delineation was then rigidly transferred to the frame of reference of the AFI image.](/cms/asset/80934ca8-3647-4a3e-8572-eefc13b37ae5/ihyt_a_1806361_f0002_c.jpg)
Figure 3. Histograms of DL-based conductivity in pelvic tissues. Conductivity histograms are reported for each tissue along with the median value (black dashed line). Conductivity histograms were based on conductivity values within manually delineated tissue ROIs, depicted on the MR magnitude image (bladder: yellow; bone: blue; fat: magenta; muscle: green; tumor: red).
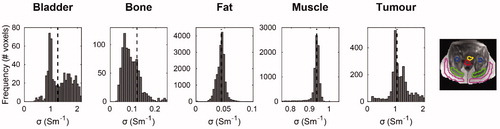
Figure 4. Vessel definition on MR and CT scans. Vessel delineations are shown on the transverse and coronal cross-sections of water image generated with the DIXON method (left) and on the HT-CT scan (right). These delineations were manually adjusted after MR to CT registration (not shown). At the bottom, a 3D rendering of the final vessel tree is shown.
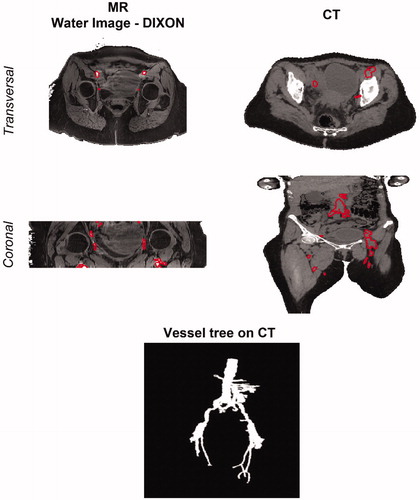
Figure 5. Patient model, SAR and Temperature maps. Transverse, sagittal and coronal cross-sections of the segmented model (first row), showing all the tissues included in the patient model, the SAR distribution (second row) and the temperature distribution (third row) in the patient. The GTV contour is indicated in black.
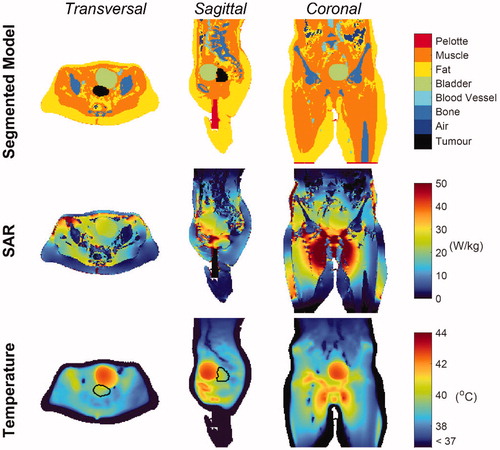
Figure 6. Equivalent dose distributions in radiotherapy and radiotherapy plus hyperthermia. Transverse, sagittal and coronal cross-sections of the radiotherapy (RT) dose distribution (first row), the temperature distribution obtained from hyperthermia treatment planning (second row), and the equivalent dose distribution derived from combining radiotherapy and hyperthermia (third row). The equivalent radiation dose was calculated within the GTV, which is denoted with blue contours. NB: The high-dose region adjacent to the GTV reflects an additional lymph node boost (with mean dose of 54.59 Gy).
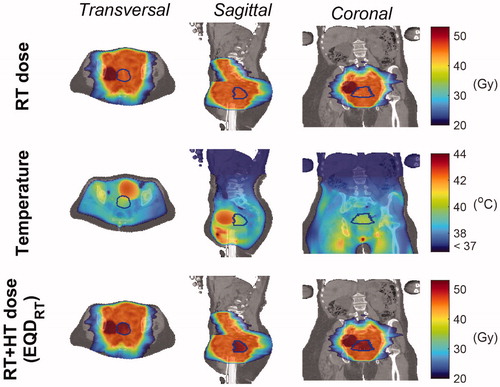
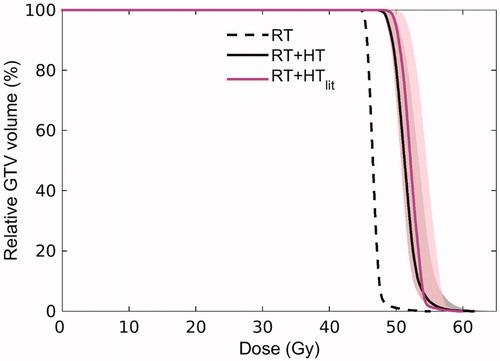
Figure 7. Dose Volume Histogram (DVH) reflecting the dose distribution in the GTV for radiotherapy alone (RT, dashed black line) and the combined radiotherapy and hyperthermia treatment as predicted with the advanced HTP workflow (RT + HT, solid black line). For comparison, the DVH predicted using a routine HTP workflow based on literature dielectric properties is also shown (RT + HTlit, solid magenta line). The shaded area around the DVH for RT + HT and RT + HTlit corresponds to confidence intervals resulting from uncertainty in LQ parameters.