Figures & data
Figure 1. Flowchart of the study. HCC: hepatocellular carcinoma; CEUS: contrast-enhanced ultrasound; RFA: radiofrequency ablation; FAA: feeding artery ablation.
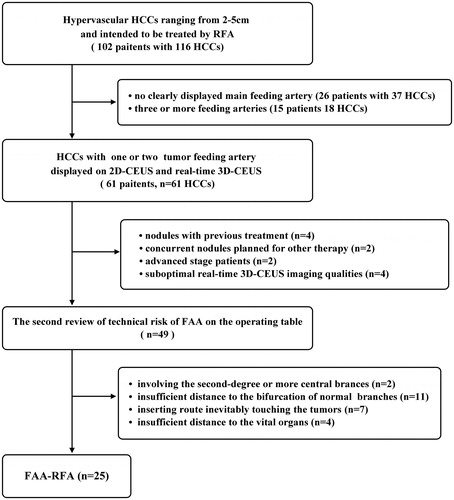
Figure 2. The practical technique of FAA as add on to RFA. FAA was performed by ablating the target feeding artery but sparing the non-feeding branches, and after that RFA was performed to ablate the tumor. FAA: feeding artery ablation.
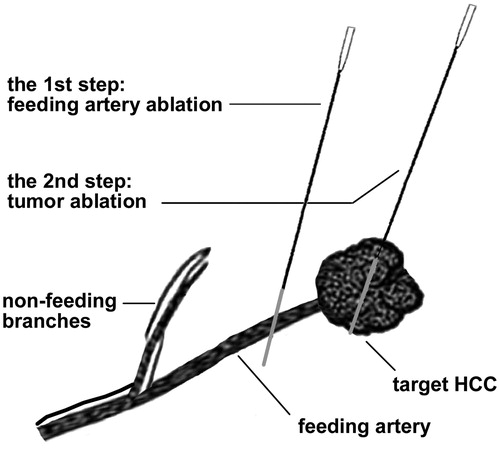
Figure 3. The examples illustrated the selection of target feeding arteries. (A) One subsegmental feeding artery (arrowhead) of the tumor (asterisk) was clearly depicted in the early arterial phase of 2D-CEUS, which is typically suitable for FAA. The principle of both sparing the non-feeding branches (fine arrow) and non-contact to the tumor could be achieved. (B) The tumor (asterisk) was not selected for FAA because of insufficient distance to the bifurcation of feeding (arrowhead) and non-feeding branches (arrow). (C) two subsegmental feeding arteries (arrowhead and thick arrow) of the tumor (asterisk) were selected as the targets of FAA, which were partly visible on 2D-CEUS through back-and-forth breathing movement. (D) Real-time 3D-CEUS depicted the two feeding arteries (arrowhead and thick arrow) in the reconstructed image with good continuity and contributed to feasibility determination. FAA: feeding artery ablation; CEUS: contrast-enhanced ultrasound.
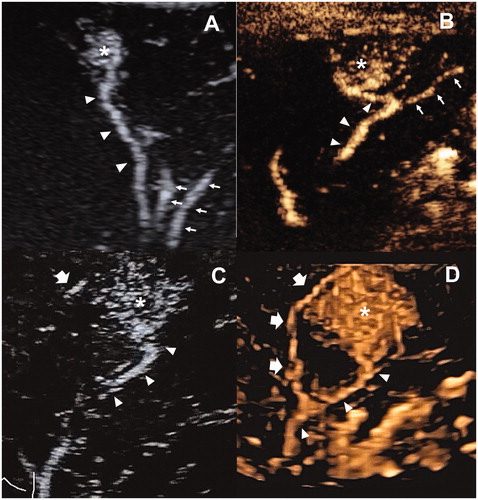
Figure 4. Images in a 41-year-old male with recurrent HCC measured 3.2 cm in diameter. (A) one feeding artery (arrowhead) of the tumor (asterisk) was depicted in the early arterial phase of 2D-CEUS. (B) FAA created a small ablation zone (arrowhead) using 1 cycle and 4 min cauterization mode. The target tumor was generally intact (asterisk). The arrow showed the electrode inserting path. (C) Immediate 2D-CEUS after FAA showed complete non-enhancement within the contour of the tumor in the arterial phase, which was identified as a complete perfusion response to FAA. (D) Hyperechoic area covered the tumor (arrowhead) after RFA, using two electrodes with a switch controller, 16 min in total. (E) 2D-CEUS performed in 30 min after RFA showed a large ablation zone measuring 5.2 cm × 4.2 cm without residue. FAA: feeding artery ablation; CEUS: contrast-enhanced ultrasound.
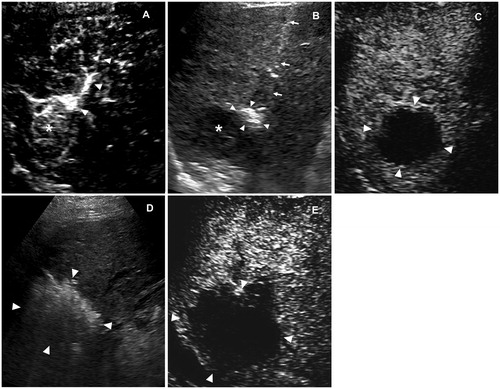
Figure 5. Images in a 43-year-old male with recurrent HCC measured 2.8 cm in diameter. (A) one feeding artery (arrowhead) of the tumor (cross) was depicted in the early arterial phase of 2D-CEUS. (B) Before FAA, 2D-CEUS showed hyperenhancement of the entire tumor at 23 s. (C) FAA created a small ablation zone (arrowhead) using 2 cycles and 8 min cauterization mode. The target tumor was intact (cross). The arrow showed the electrode inserting path. (D,E) After FAA, dual imaging showed intratumoral enhancement defect of the tumor at 23 s, which identified as a partial perfusion response to FAA. FAA: feeding artery ablation; CEUS: contrast-enhanced ultrasound.
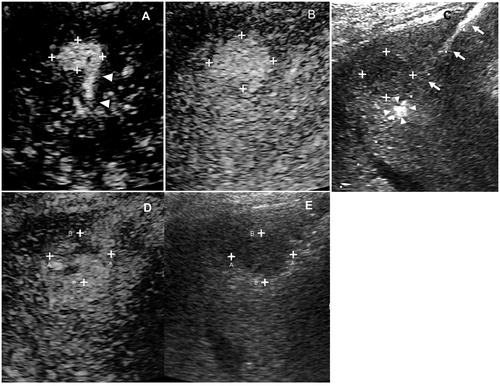
Table 1. Demographic and Baseline Clinical Characteristics of Patients.
Table 2. Technical details of FAA as add-on to RFA in 25 tumors with 29 target feeding arteries.
Table 3. Comparison of ablation sizes according to perfusion reduction (complete vs. partial response).
Figure 6. Follow-up of FAA-RFA. (A) cumulative rate of LTP, (B) cumulative overall survival and (C) cumulative recurrence-free survival. LTP: local tumor progression; FAA-RFA: feeding artery ablation-radiofrequency ablation.
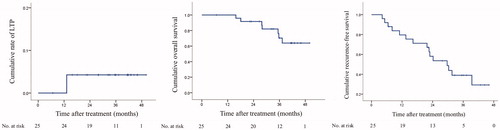
Table 4. Univariate and multivariate cox analysis of the predictors of overall survival.
Table 5. Univariate and multivariate Cox analysis of the predictors for recurrence-free survival.
