Figures & data
Figure 1. RF ablation (RFA) of local tumor accelerates distant tumor growth. (A) Curves for MC38 tumors demonstrate an identical rate of growth for all groups before treatment After partial treatment with RFA (day 1), the tumor growth rate is significantly greater such that tumor diameter is significantly greater (p < 0.01) than that with sham treatment. (B) Curves for CT26 tumors demonstrate an identical rate of growth for all groups before treatment. After partial treatment with RFA (day 3), the tumor growth rate is significantly greater such that tumor diameter is significantly greater (p < 0.01) than that with sham treatment. (C,D) Immunohistochemical (IHC) staining for ki-67 demonstrating cell proliferation. RFA increased cell proliferation compared to sham treatment for MC38(C), and CT26 (D). (E,F) IHC staining for CD34 demonstrating microvascular density. RFA increased cell microvascular density compared to sham treatment for MC38 (E), and CT26 (F). G. Curves for R3230 tumors demonstrate identical growth rates for all groups before treatment. After tumor RFA (day 0), growth distant tumors is significantly greater such that tumor diameter is significantly greater than that with sham treatment.
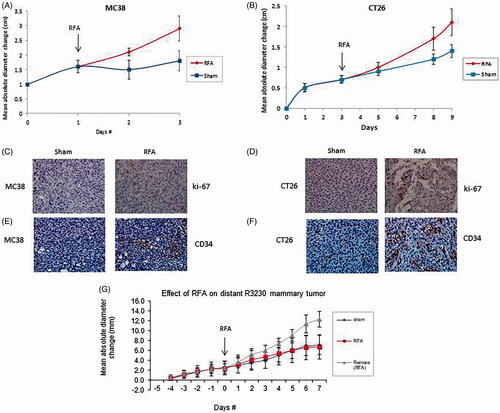
Table 1. Tumor growth and proliferative index for rodent tumors post RFA.
Figure 2. PHA reduces post-RFA c-Met expression in the periablational rim. (A) Immunohistochemical staining for c-Met receptor demonstrating c-Met expression in RFA periablational rim (arrowheads) vs. RFA with PHA 7 days post treatment. PHA addition to RFA reduces significantly c-Met expression (to minimal expression – arrowheads) compared to RFA alone. (B) Bar chart quantifying this infiltration of c-Met receptor + cells into the tumoral periablational rim 7 days post RFA. There is a significant reduction of c-Met positive cells in tumor tissue post RFA treated with PHA compared to RFA alone. (C,D) Western blot assays demonstrate increased c-Met receptor protein in the periablational tissue surrounding the ablation zone from pooled samples compared with sham treatment (seen as dense bands after gel electrophoresis at 50-kDa level, where the c-Met receptor a subunit is expected, after b-actin standardization). c-Met receptor protein levels are also increased in the distant untreated tumor after RFA compared with sham treatment. Addition of PHA reduces c-Met receptor protein levels in both local and distant tumor. (A431: Positive control; ST: Sham tumor; RFT: RFA tumor; RPT: RFA + PHA tumor; SRT: Sham remote tumor; RFRT: RFA remote tumor; RPRT: RFA + PHA remote tumor).
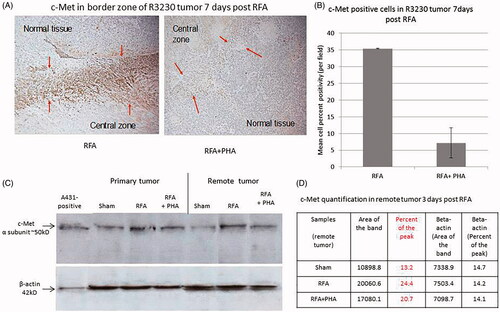
Table 2. Serologic cytokine expression for rodent tumors post RFA.
Figure 3. Inhibitors suppress distant tumor growth after local tumor RFA. (A) Graph of tumor growth of local and distant tumor with or without PHA treatment demonstrates the effect of tumor RFA on distant tumor growth in R3230 adenocarcinoma cell line in the presence of a c-Met kinase inhibitor. Graph shows that adjuvant PHA given 3 days after RFA decreases distant R3230 tumor growth rate back to baseline compared with RFA alone. PHA alone treatment arm has same growth rate as sham arm indicating that c-Met inhibitor does not have active effect on tumor growth by itself. B. Effect of tumor RFA on distant tumor growth in R3230 adenocarcinoma cell line with suppression of VEGF receptor inhibitor. Similar to PHA, anti-VEGF blocks R3230 tumor growth back to baseline compared to sham.
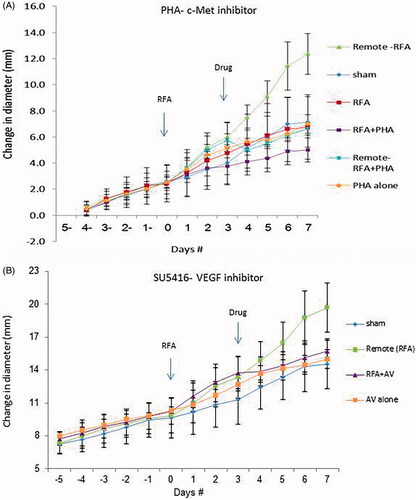
Figure 4. PHA reduces cell proliferation following RFA. Immunohistochemical staining for ki-67 demonstrating cell proliferation. Tumor RFA increases nuclear staining, with PHA treatment reducing cell proliferation to sham level 7 days post RFA.
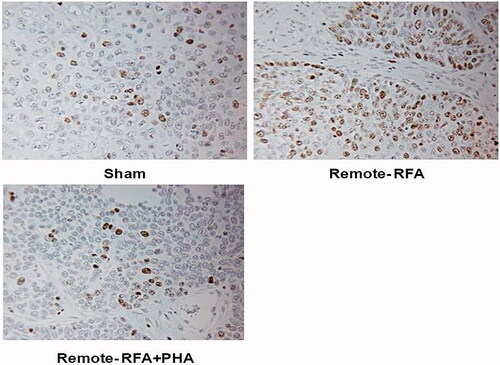
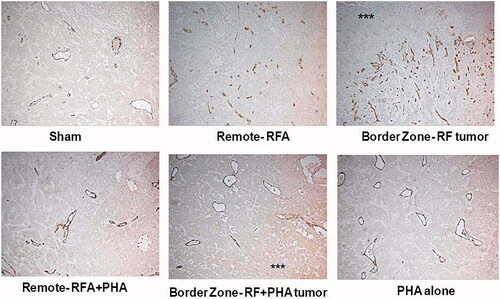
Figure 5. PHA reduces microvascular density levels following RFA. Microvascular density for RFA periablational rim vs RFA with PHA 7 days post treatment. Immunohistochemical staining for CD34 demonstrating increases in microvascular density post-RFA in local periablational rim and distant tumor. Addition of PHA to RFA reduced microvascular density levels to that similar to sham. ***Ablated zone adjacent to the periablational zone.