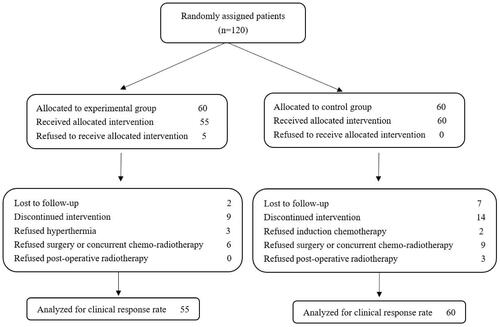
Figures & data
Figure 1. (A) Ultrasonic thermal therapy system; (B) Representative CT scan of OSCC patients pre- and post-hyperthermia therapy; (C) Representative section of hematoxylin and eosin staining post-hyperthermia therapy in OSCC patients.
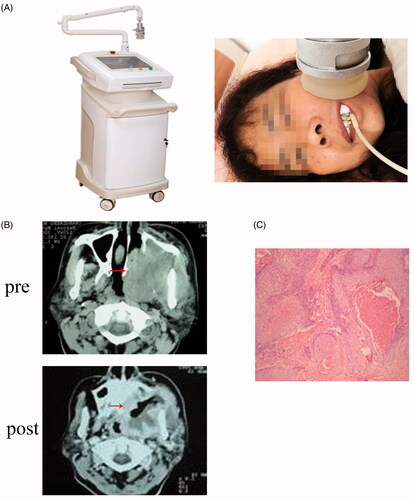
Table 1. Baseline patient demographic and clinical characteristics.
Table 2. Clinical response of hyperthermia.
Figure 3. (A) Overall and (B) disease-free survival in the control and experimental arms. TPF: docetaxel, cisplatin, and fluorouracil.
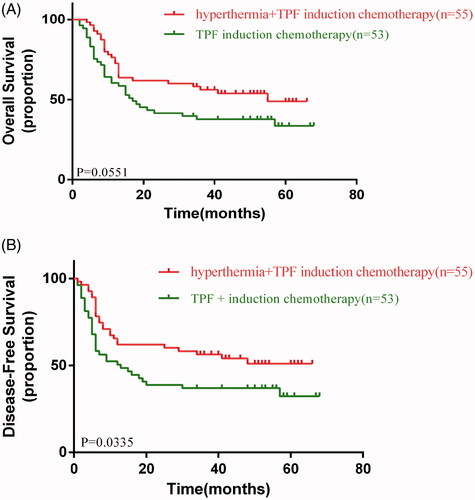
Table 3. Toxicity.
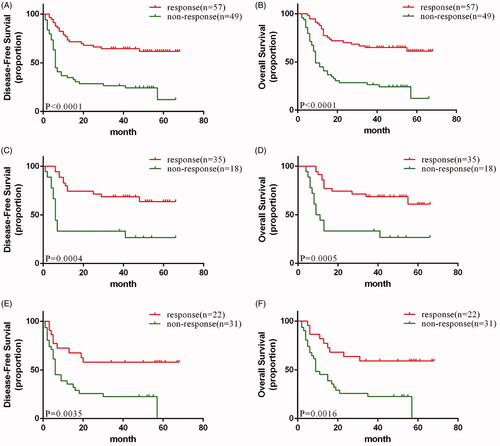
Figure 4. (A) disease-free and (B) Overall survival of response and non-response patients in total population; (C) disease-free and (D) Overall survival of response and non-response patients in experimental arm; (E) disease-free and (F) Overall survival of response and non-response patients in control arm.