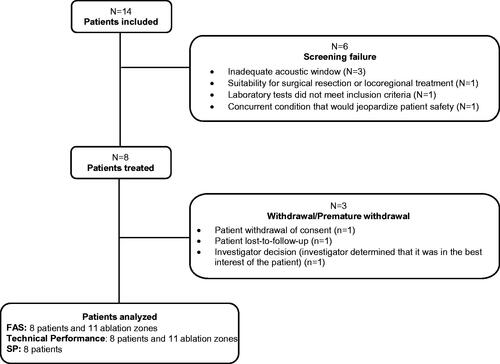
Figures & data
Table 1. Patient and tumor characteristics.
Table 2. Histotripsy ablation procedure.
Figure 2. Representative qEASL image. Contrast-enhanced MRIs obtained at baseline (A) and 24 hours (B), 1 week (C), 1 month (D), and 2 months (E) posttreatment in a patient with diffuse colorectal liver metastases who had undergone multiple types of therapy including surgical resection and several lines of systemic chemotherapy. A small lesion in segment 2 measuring 1 cm in diameter was treated with histotripsy. The three-dimensional measurement known as qEASL (quantifiable European Association for the Study of Liver) was performed using a semiautomatic tumor segmentation software (Philips Healthcare). The qEASL algorithm quantified the tumor diameter, absolute tumor volume, and enhancing tumor volume as absolute numbers as well as the percentage of the enhancing tumor volume. In a previous radiology-histopathology study, excellent correlation between the appearance on MR imaging and the findings at pathology both in terms of tumor viability (enhancing on MRI) and necrosis (lack of enhancement on MRI) confirmed the usefulness of qEASL mapping to assess tumor response [Citation22,Citation23].
![Figure 2. Representative qEASL image. Contrast-enhanced MRIs obtained at baseline (A) and 24 hours (B), 1 week (C), 1 month (D), and 2 months (E) posttreatment in a patient with diffuse colorectal liver metastases who had undergone multiple types of therapy including surgical resection and several lines of systemic chemotherapy. A small lesion in segment 2 measuring 1 cm in diameter was treated with histotripsy. The three-dimensional measurement known as qEASL (quantifiable European Association for the Study of Liver) was performed using a semiautomatic tumor segmentation software (Philips Healthcare). The qEASL algorithm quantified the tumor diameter, absolute tumor volume, and enhancing tumor volume as absolute numbers as well as the percentage of the enhancing tumor volume. In a previous radiology-histopathology study, excellent correlation between the appearance on MR imaging and the findings at pathology both in terms of tumor viability (enhancing on MRI) and necrosis (lack of enhancement on MRI) confirmed the usefulness of qEASL mapping to assess tumor response [Citation22,Citation23].](/cms/asset/bd32d144-7a05-48a8-a6a7-4e8d7a824aec/ihyt_a_2112309_f0002_c.jpg)
Figure 3. Involution of treatment zone. Tumor and treatment zone (red arrow) at each time point on contrast-enhanced ultrasound.
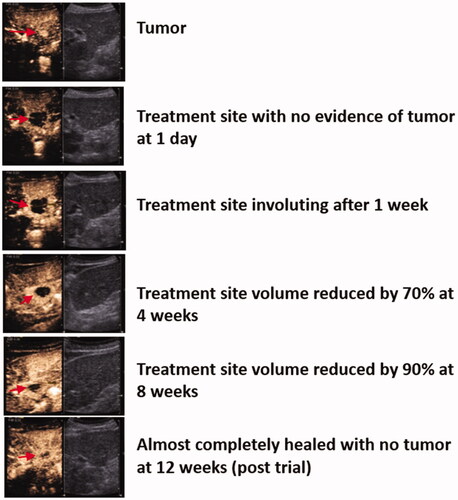
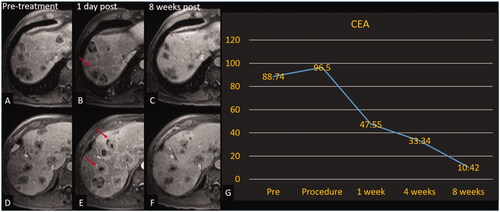
Figure 4. Histotripsy of CRLM with decreasing CEA. Patient with colorectal cancer with involution of the non-treated tumors. Treated tumor is not shown. Axial contrast-enhanced MR images obtained at 2 different slices; pretreatment (A, D), 1-day post treatment (B, E), and 8-week post-treatment (C, F). Red arrows (B, E) denote tumors with increased peripheral enhancement (reaction around off-target tumors that could indicate a systemic anti-tumor response) compared with pretreatment images. (G) Graph of CEA over the course of the trial for this patient.