Figures & data
Figure 1. Preparation of a cystic neoplasm mimic model. (A) The VX2-implanted tumor bladder showed that the bladder mucosa was smooth, the blood vessels were clearly visible on the surface, and the VX2 tumor (yellow arrow) was located in the anterior wall of the bladder. (B) The VX2-implanted tumor bladder was immersed in agarose solution that had been cooled to 40–45 °C and kept intact in the solution. The gel fixation matrix was formed by leaving it at room temperature (23 °C) for approximately 40 min to complete the development of cystic neoplasm mimic models.
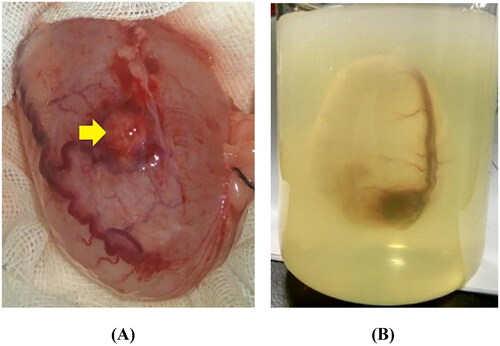
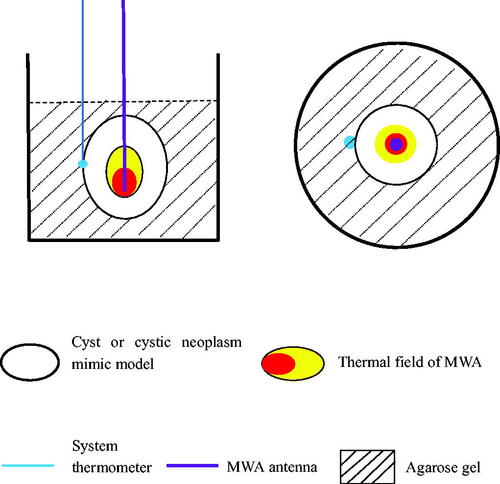
Figure 4. CT imaging of the MWA procedure. (A) The cyst mimic model showed a regular morphology with clear borders after contrast injection into the cystic cavity (yellow arrow). (B) The cystic neoplasm mimic model showed a regular morphology with clear borders (red arrow). The filling defect of the VX2-implanted tumor (yellow arrow) was visible in the cystic cavity after contrast injection. (C) A 17 G microwave antenna (red arrow) was inserted along the length of the cystic neoplasm mimic model, perpendicular to the level of the agarose gel fixation base. (D) The system thermometer (yellow arrow) was placed at the outer edge of the bladder wall to monitor temperature changes.
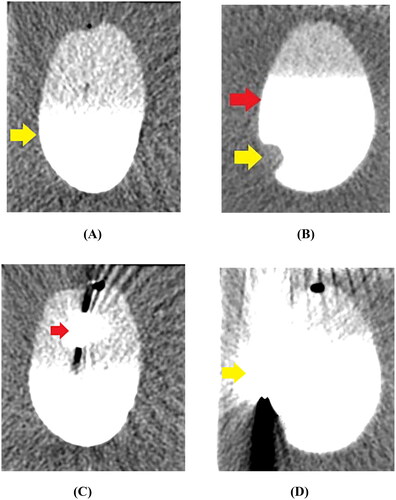
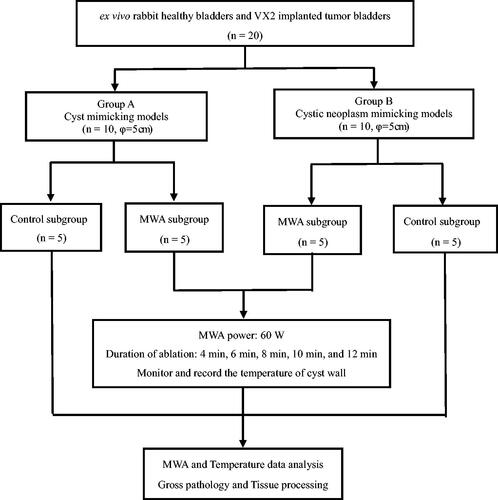
Table 1. MWA experimental group with cyst mimicking models (Power/Transverse diameter: 60 W/5 cm) (Mean ± SD, °C)
Table 2. MWA experimental group with cystic neoplasm mimic models (Power/Transverse diameter: 60 W/5 cm) (Mean ± SD, °C)
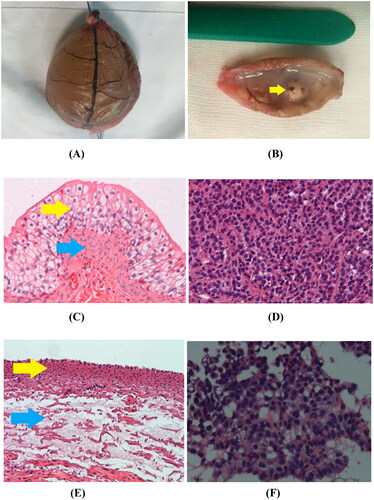
Figure 5. Gross specimen and pathological examination imaging. (A) Gross morphology image of ex vivo rabbit healthy bladder treated with MWA after 12 min duration showed that the blood vessels in the bladder wall appeared withered and looked like dendritic branches, and the bladder surface appeared dark brown. (B) Gross morphology image of VX2 implanted tumor (yellow arrow) treated with MWA after 12 min duration appeared grayish white and soft. (C) In the control group, 100× H&E staining showed that the integrity of the mucosal structure and the epithelial cells (yellow arrow) were maintained, and the lamina propria and muscularis (blue arrow) were intact. (D) In the control group, H&E staining of the VX2 tumor showed irregular tumor cell morphology, large deep-stained nuclei, obvious atypia, and high mitotic rates. (E) Immediately after MWA, 100× H&E histology showed that complete shedding of mucosal epithelial cells of the bladder wall, necrosis of the muscularis mucosa (yellow arrow), and the loss of histological patterns and structures in all layers of the bladder wall (blue arrow). (F) Immediately after MWA, 100× H&E histology showed coagulative necrosis in most of the VX2 tumor cells, along with karyopyknosis and deeply stained cytoplasm.
Data availability statement
No data was used for the research described in the article.