Figures & data
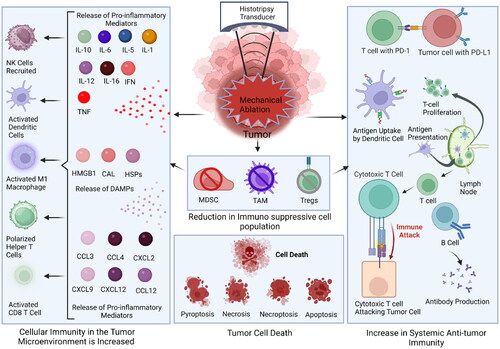
Figure 1. Schematic of histotripsy mediated immune system modulation. Histotripsy significantly alters the tumor microenvironment through the elimination of immunosuppressive cells in the ablation zone. Likewise, cells adjacent to the ablation zone that are damaged may undergo a range of cell death processes, including inflammatory cell death and apoptosis. The reduction in immunosuppression appears to be countered by an increase in a selection of damage associated molecular patterns and the production of inflammatory mediators, including cytokines and chemokines. Together, these changes shift the immunologically ‘cold’ tumor microenvironment toward one that is more pro-inflammatory and ‘hot’. This is characterized by an influx of inflammatory cells into the tumor microenvironment. It has been postulated that histotripsy treatment generates higher quality and quantity antigens. The improved antigen presentation has been suggested to augment the systemic, anti-tumor immune response, which has been characterized by an increase in cytotoxic T cell recruitment to local and distal tumors and reduced metastatic burden. Ultimately, these changes may improve immunotherapy responsiveness.