Figures & data
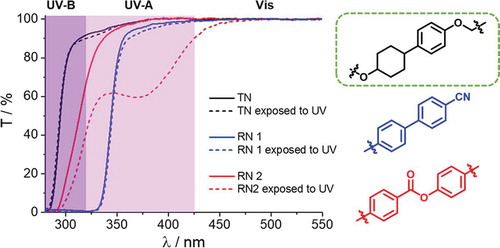
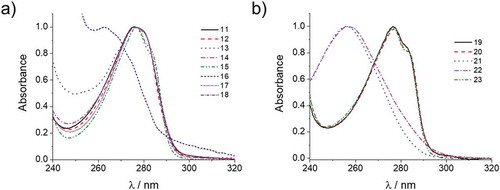
Table 1. Summary of phase behaviour, enthalpy of LC phase transition and maximum absorption wavelength (λmax) of the synthesised reactive mesogens. Monotropic LC phases are indicated in the brackets
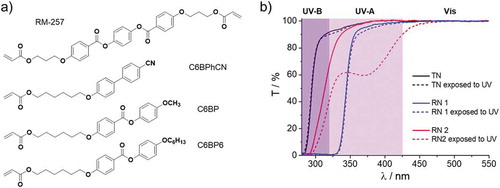
Table 2. Chemical compositions (in wt.%) of the test network (TN) and reference networks (RN1 and RN2) and their total transparencies in different UV spectral regions
Figure 1. Synthesis of compounds 1, 2, 3, 12, 13 and 14. i: K2CO3, KI, 6-bromohexanol, EtOH, reflux 48 h; ii: NEt3, acryloyl chloride, THF, 0°C 1 h, r.t. 16 h
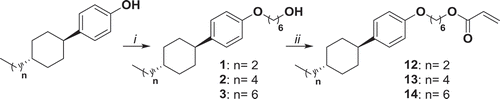
Figure 2. Synthesis of compound 15. i: K2CO3, KI, bromohexane, EtOH, reflux 48 h; ii: 6-bromohexanoyl chloride, NEt3, THF, 0°C 1 h, r.t. 16 h; iii: potassium acrylate, KI, DMSO, 52°C 72 h
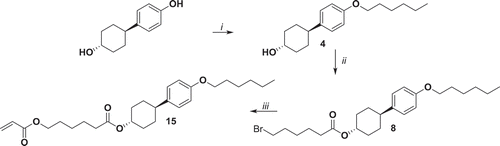
Figure 3. Synthesis of compound 16. i: NaBH4, MeOH, r.t. 90 min. ii: 6-bromohexanoyl chloride, NEt3, THF, 0°C 1 h, r.t. 16 h; iii: potassium acrylate, KI, DMSO, 52°C 72 h
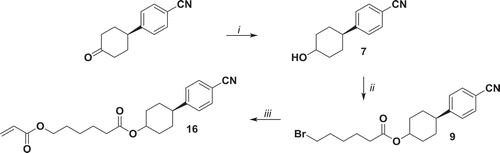
Figure 4. Synthesis of compound 17. i: K2CO3, KI, 1,6-dibromohexane, EtOH, reflux 48 h; ii: potassium acrylate, KI, DMSO, 52°C 72 h; iii: hexanoyl chloride, NEt3, THF, 0°C 1 h, r.t. 16 h
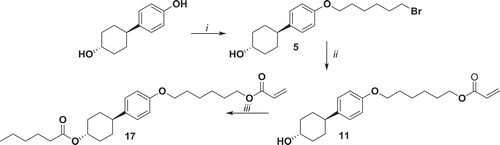
Figure 6. Synthesis of 19. i: K2CO3, KI, 1,6-dibromohexane, EtOH, reflux 48 h; ii: 6-bromohexanoyl chloride, NEt3, THF, 0°C 1 h, r.t. 16 h; iii: potassium acrylate, KI, DMSO, 52°C 72 h
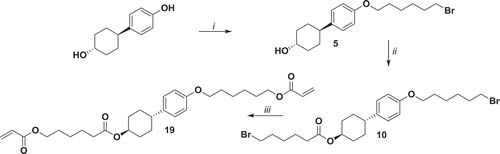
Figure 7. Synthesis of 20. i: NaH, 1,6-dibromohexane, THF reflux 78 h; ii: potassium acrylate, KI, DMSO, 52°C 72 h
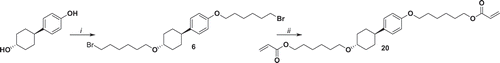
Figure 8. Synthesis of 21, 22 and 23. i: DCC, DMAP, 4-((6-(acryloyloxy)hexyl)oxy)benzoic acid, CH2Cl2, 30°C 24 h. ii: 4-((6-(acryloyloxy)hexyl)oxy)-2-methylbenzoic acid, triethylamine, 2,4,6-trichlorobenzoyl chloride, DMAP THF 18 h r.t.; iii: NEt3, acryloyl chloride, THF, 0°C 1 h, r.t. 16 h
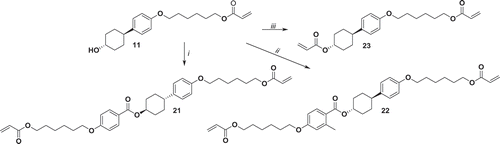
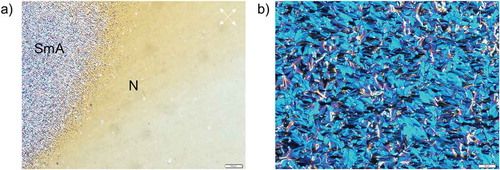
Figure 9. Polarised optical microscopy images of LC textures of compound 14 demonstrating the transition from smectic A to nematic (a) upon cooling, and fan-like texture of smectic A obtained at 22°C (b). Planar boundary conditions. Scale bars correspond to 100 and 50 µm, respectively. Polariser and analyser are shown as white arrows