Figures & data
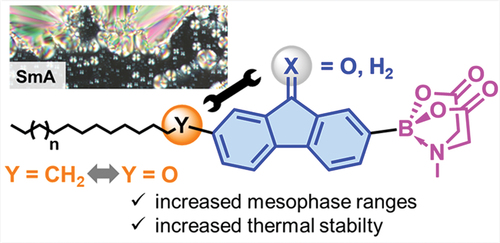
Scheme 1. Overview of preliminary work on liquid crystalline fluorenes, fluorenones and MIDA boronates.
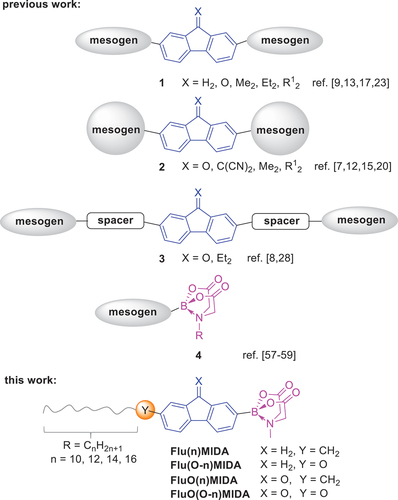
Scheme 2. Synthesis of the fluorene and fluorenone MIDA boronates Flu(n)MIDA, FluO(n)MIDA with alkyl side chains.
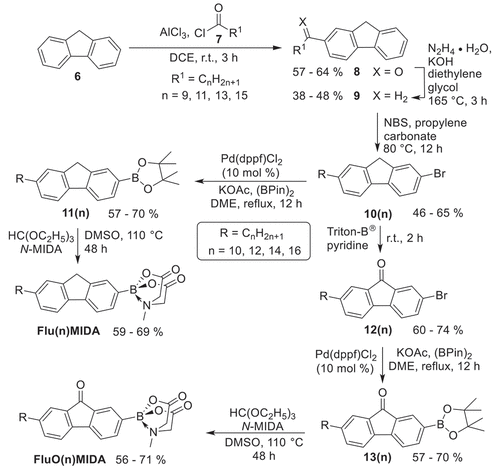
Scheme 3. Synthesis of the alkoxy-substituted fluorene or fluorenone MIDA boronates Flu(oO-n)MIDA, FluO(O-n)MIDA.
Figure 1. (Colour Online) POM images of 17(O-10) at (a) 82°C and (b) 45°C and (c) 17(O-12) at 85°C and (d)75°C (200 × magnification, taken upon cooling from the isotropic phase (5 K · min−1).
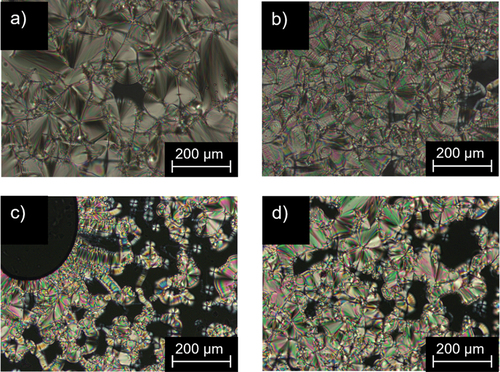
Table 1. Phase transition temperatures (in °C) and phase transition enthalpies (in kJ/mol) of 17(O-n). a,b.
Figure 3. (Colour Online) (a) SAXS profile and (b) WAXS profile of 17(O-12) at 57°C (black) and 85°C (blue).
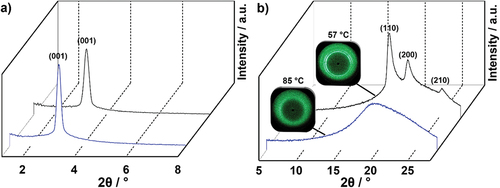
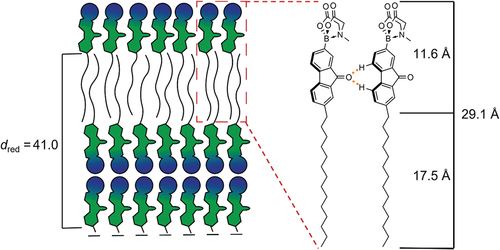
Figure 4. (Colour Online) Proposed packing model of the SmE phase of 17(O-12) in (a) top view, (b) side view and the proposed packing model of the SmA bilayer (c).
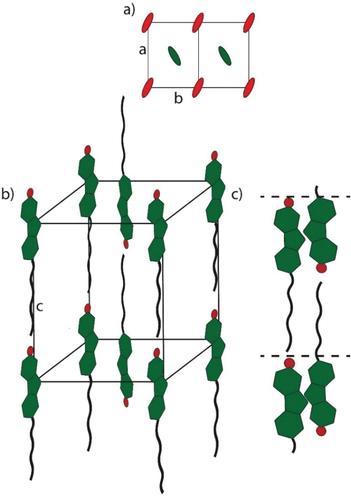
Table 2. X-ray diffraction data of bromoalkoxyfluorene 17(O-12). The measurements were performed during cooling from the isotropic liquid phase. The halo was determined from WAXS.
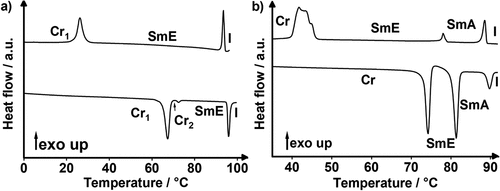
Figure 5. (Colour Online) DSC traces of (a) Flu(12)MIDA, (b) Flu(O-12)MIDA, (c) FluO(12)MIDA (phase transitions (Tg) determined via POM due to the absence in DSC traces) and (d) Flu(O-12)MIDA; 1st heating/cooling of (a-c) 10 K · min−1 and 1st, 2nd, 3rd heating/cooling of d), 10 K · min−1).
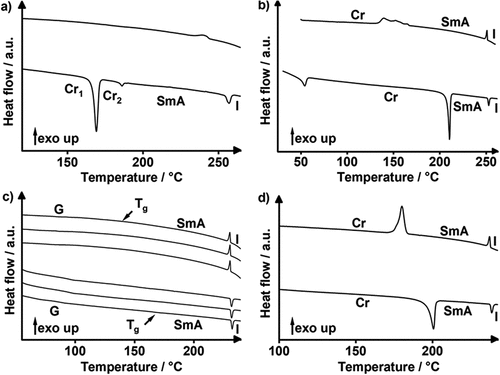
Figure 6. (Colour Online) Results from DSC measurements (rounded values, 1st heating cycle; rate 10 K·min− 1) of Flu(n)MIDA, Flu(o-n)MIDA, FluO(n)MIDA and FluO(O-n)MIDA. Gray: crystalline phase; blue: smectic a phase.
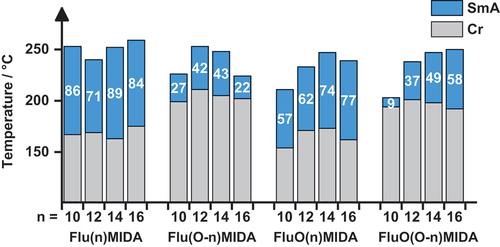
Table 3. Phase transition temperatures T (in °C) and phase transition enthalpies (in kJ/mol) of Flu(n)MIDA, FluO(n)MIDA, Flu(o-n)MIDA and FluO(O-n)MIDA.a,b
Figure 7. Out-of plane and in-plane conformations of alkyl and alkoxy chains on six-membered aromatic rings. The figure was adapted from ref [Citation85].
![Figure 7. Out-of plane and in-plane conformations of alkyl and alkoxy chains on six-membered aromatic rings. The figure was adapted from ref [Citation85].](/cms/asset/8fe115ec-29de-4c87-baed-167cb5a6018d/tlct_a_2319626_f0007_b.gif)
Figure 8. (Colour Online) POM images of (a) Flu(12)MIDA at 220°C; (b) Flu(O-12)MIDA at 228°C; (c) FluO(12)MIDA at 225°C; (d) FluO(O-12)MIDA at 229°C (200 x magnification, taken upon cooling from the isotropic phase (5 K · min−1).
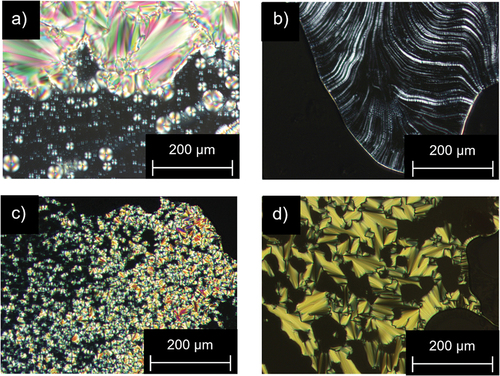
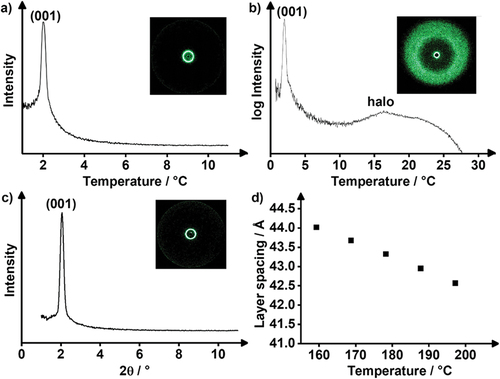
Table 4. X-ray diffraction data for fluorene Flu(12)MIDA and fluorenone FluO(14)MIDA. The measurements were performed during cooling from the isotropic liquid phase. The halo was determined from WAXS.
Figure 9. (Colour Online) (a) SAXS profile and (b) WAXS profile of Flu(12)MIDA and (c) SAXS profile and (d) temperature-dependant layer spacing of FluO(14)MIDA with the corresponding 2D-pattern. (WAXS profile see ESI fig. S9).