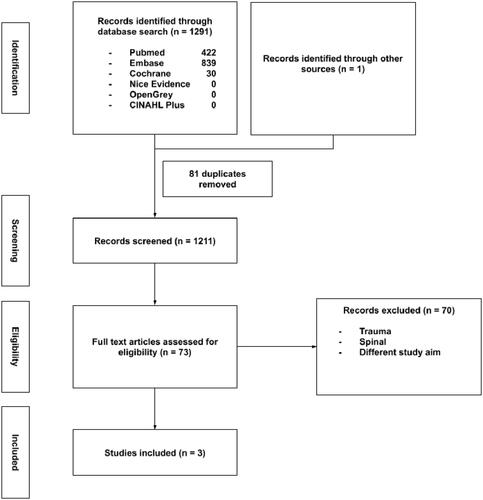
Figures & data
Table 1. Summary of major national guidelines for pharmacological VTE prophylaxis in cranial neurosurgery.
Table 2. Characteristics of eligible studies included in this systematic review.
Figure 2. Risk of bias, determined according to the Cochrane ROBINS-I tool. Risk of bias in each domain is classed as serious, moderate, low or no information as seen in the legend. VTE: venous thromboembolism; ICH: intracranial haemorrhage; D1: bias due to confounding; D2: bias in selection of participants into study; D3: bias in classification of interventions; D4: bias due to deviations from intended intervention; D5: bias due to missing data; D6: bias in measurement of outcomes, D7: bias in selection of reported result.
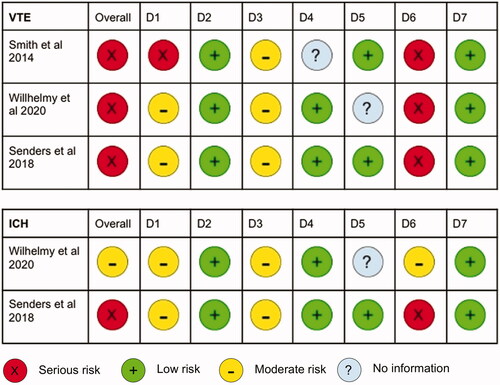
Table 3. VTE and ICH Rates and time of initiating prophylactic anticoagulation after surgery.