Figures & data
Figure 1. Laboratory experiment system containing the microcosm. After passing through dilute sulfuric acid to remove the ammonium, air (0.1 L min−1) was blown across the water surface and bubbled through 2% boric acid to absorb NH3 emitted from the experimental system. Sampling outtake was used to collect the gas sample for NO, N2O and N2.
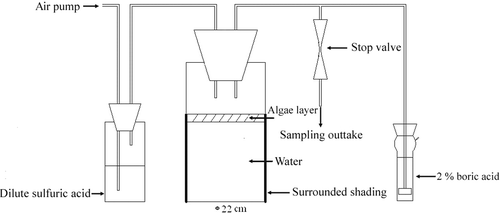
Table 1. The physical and chemical characteristics of water collected from Lake Taihu.
Table 2. The composition of algae (based on cell numbers) used in experiments.
Figure 2. Temporal variations of nitrate, nitrite and dissolved oxygen concentrationsin submerged tanks with 4 × 109 cells L−1 cyanobacteria added and control tanks in the field experiment. Data are means ± SD (n = 3).
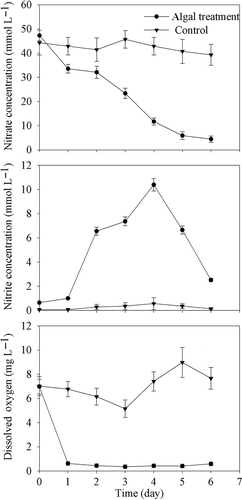
Figure 3. The variations of DO and pH in the water column of Meiliang Bay from 22:00 h to 12:00 on 3 days. Data are the means ± SD (n = 3).
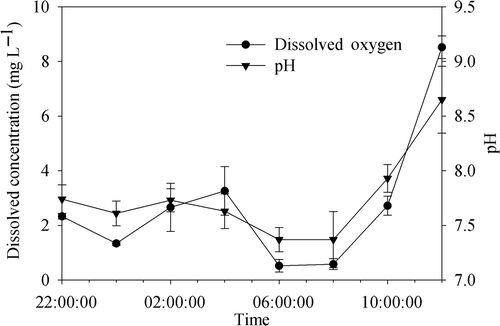
Figure 4. Temporal variation of pH (a) and dissolved oxygen (b) in laboratory microcosms with different cyanobacterial densities. Data are means ± SD (n = 3).
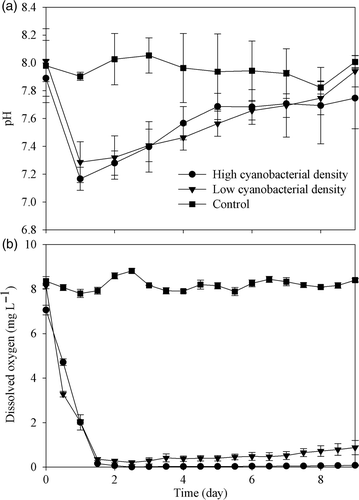
Table 3. The initial and final concentrations of different nitrogen compounds and 15N isotopic abundances obtained from a laboratory incubation of spiked lake water for 9 days. The two treatments were addition of 2 × 109 cells L−1 and 4 × 109 cells L−1 of cyanobacteria.
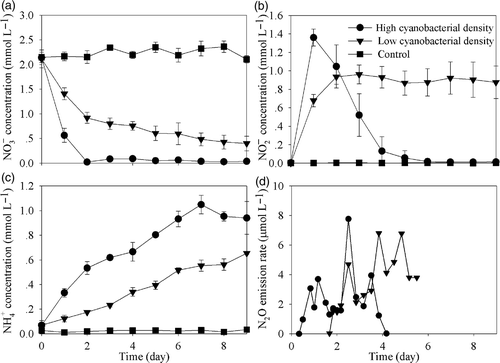
Figure 5. Temporal variation of concentrations of (a),
(b),
(c), and N2O emission rates (d) in microcosms with different cyanobacterial densities. Data are means ± SD (n = 3).