Figures & data
Table 1. Baseline subject demographics.
Figure 1. Investigator-evaluated ocular redness scores (0–4 scale) before and after instillation of brimonidine tartrate ophthalmic solution, 0.025% or its vehicle at Day 0 (Visit 1). Data are the mean (SD) for the ITT population with last observation carried forward.
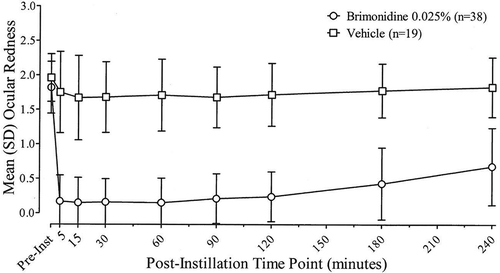
Figure 2. Percentage of subjects with total clearance of ocular redness at Day 0 (Visit 1) following instillation of brimonidine tartrate ophthalmic solution, 0.025% or its vehicle.
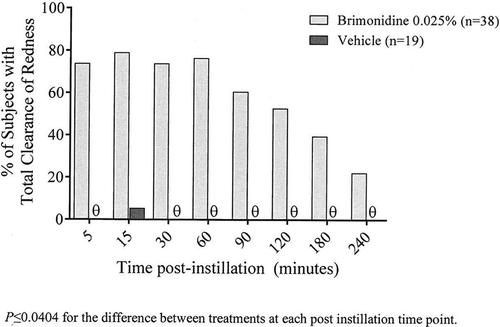
Table 2. Ocular redness (Mean [SD]) – subject diaries – ITT population with last observation carried forward.
Figure 3. Average daily post-instillation ocular redness scores (0–4 scale) based on subject diary data during treatment (Days 0 to 28) and for seven days following treatment discontinuation (Days 29 to 35). Data are the mean (SEM) for the ITT population with observed data only.
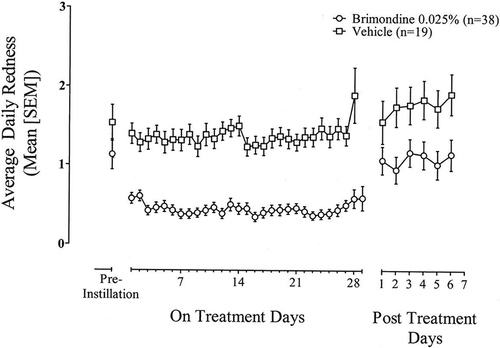
Table 3. Ocular whitening – subject diaries – ITT population with observed data only.
Table 4. Rebound redness – safety population.
Table 5. Ocular adverse events – safety population.
