Figures & data
Figure 1. Normal Appearance of the Cornea and Limbus. The healthy cornea is optically transparent (a) and surrounded by the annular limbus which separates it from the opaque sclera. The limbus contains the palisades of Vogt (b) which are finger-like projections of the stroma. The optical coherence tomography (OCT) image of the normal cornea shows a smooth uniform stratified epithelium (c) with underlying compact stroma. The OCT angiography image of the normal limbus shows linear anastomosing hair-pin loops of the limbal capillaries (d) with adjacent avascular corneal stroma.
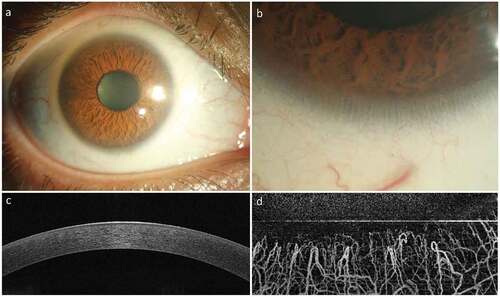
Figure 2. Clinical Appearance of Limbal Epithelial Stem Cell Deficiency (LSCD). LSCD is characterized clinically by loss of corneal clarity because of superficial neovascularization (a), positive fluorescein staining (b), presence of conjunctival cytokeratin (CK) markers on impression cytology (CK19) and immunohistochemistry (c), altered epithelial cell morphology with sub-basal fibrosis on in vivo confocal microscopy (d), replacement of the dark hyporeflective corneal epithelial phenotype with bright and thick conjunctival phenotype on optical coherence tomography (OCT, E) and loss of normal limbal vascular architecture on OCT angiography (F).
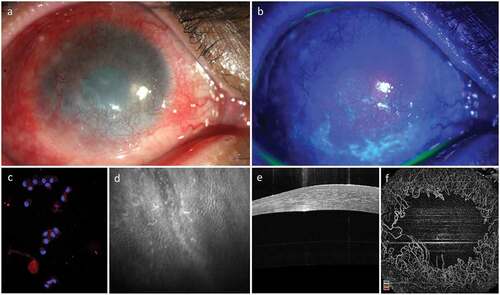
Table 1. Aetiological classification of limbal stem cell deficiency.
Table 2. Clinical outcomes of conjunctival-limbal autografting (CLAu) for unilateral limbal epithelial stem cell deficiency (LSCD).
Table 3. Techniques and clinical outcomes of autologous-cultivated limbal epithelial transplantation (CLET) for unilateral Limbal Stem Cell Deficiency (LSCD).
Figure 3. Limbal Explant Culture on the amniotic membrane: After attaching the explants to the hAM membrane, slow growth can be seen around the explant till the whole membrane is fully covered. Day 2 (a), Day 9 (b), Day 15 (c). Phenotypic marker expression by LESCs. LESCs showing positive expression of the CK3 + 12, their native phenotypic marker and ABCG2, stem cell marker, on day 9 of culture.
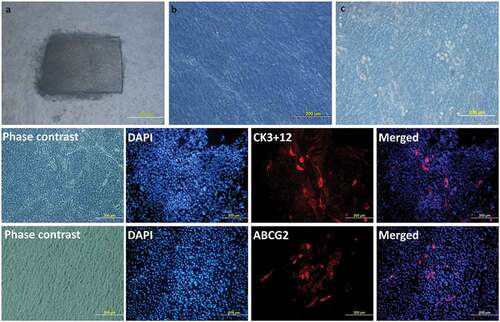
Table 4. Clinical outcomes of autologous simple limbal epithelial transplantation (SLET) for Unilateral Limbal Stem Cell Deficiency (LSCD).
Figure 4. Clinical Outcome of Simple Limbal Epithelial Transplantation (SLET). The top row shows the pre-operative appearance of affected eye with opacification and vascularization of the corneal surface (a) and a thick bright hyperreflective conjunctival epithelial phenotype seen on optical coherence tomography (OCT, b). The same eye one-year post-operative shows remarkably improved corneal clarity, absence of vascularization and opacification (c) and smooth dark hyporeflective corneal epithelial phenotype on OCT imaging (d).
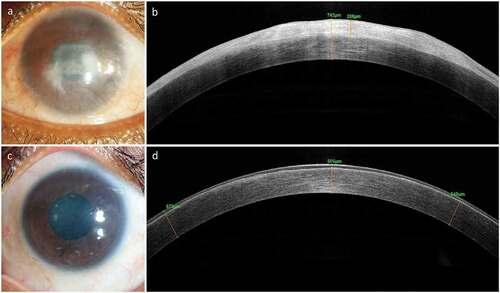
Table 5. Clinical outcomes of conjunctival limbal allografts (CLAL)/Keratolimbal allograft (KLAL) for Bilateral Limbal Stem Cell Deficiency (LSCD).
Table 6. Clinical Outcomes of Allogeneic Cultivated Limbal Epithelial Transplantation (CLET) for Bilateral Limbal Stem Cell Deficiency (LSCD).
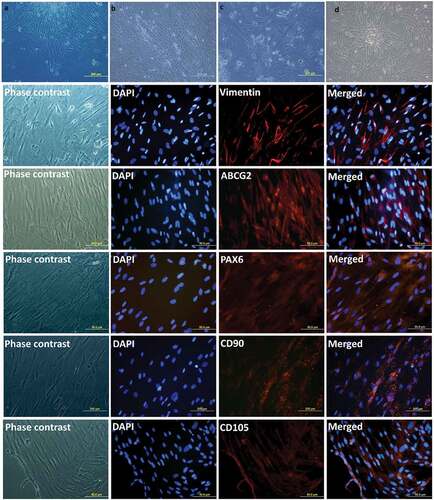
Figure 5. Expansion of Limbal Mesenchymal Stem Cells (LMSCs). Phase-contrast images of the cultured LMSCs on Day 3 (a) and Day 15 (b) of primary culture; end of passages 1 (c) and 2 (d). Phenotypic expression of LMSCs. The LMSCs show positive expression of the markers for mesenchymal-lineage: Vimentin, CD90 and CD105; and are also positive for stem cell markers ABCG2 and PAX-6.