Figures & data
FIGURE 1. Transmitted light images of transverse thin sections of Cape hyrax (Procavia capensis) left tibia and fibula by a 35 mm film/slide scanner (OpticFilm 8100, Plustek Inc.). A, the section prepared with the method developed by Urano et al. (Citation2019); B, section soaked in acetone for 1 week; and C, section soaked in Quadrol for 1 week. The asterisk (*) indicates the heme pigment distributed in the bones. The directions of bones are above, anterior; right, lateral; left, medial; and bottom, posterior. Abbreviations: ep, epoxy resin; fb, fibula; LAG, line of arrested growth; and tb, tibia. All field widths equal 10.51 mm.
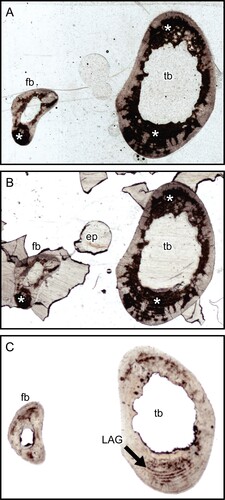
FIGURE 2. Transverse thin section of lesser Japanese mole (Mogera imaizumii) left humerus prepared by the method of Urano et al. (Citation2019: see the heading Conventional Thin Sectioning Method). A, C, E, transmitted, and B, D, F, polarized light microscopic images. The asterisk (*) indicates the heme pigment distributed in the bone. The directions of bone are above, anterior; right, medial; left, lateral; and bottom, posterior. Abbreviation: ca, canal. The field widths equal 5.59 mm (A, B), 2.15 mm (C, D), and 845 μm (E, F).
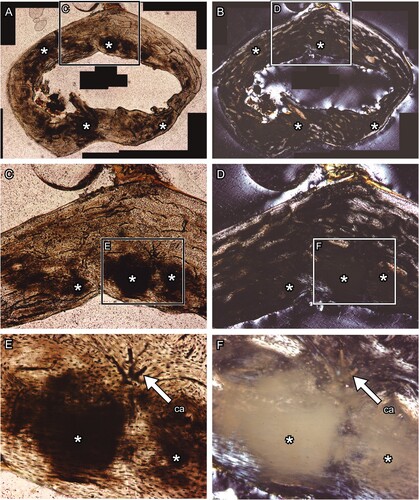
FIGURE 3. Transverse thin section of raccoon (Procyon lotor) left metacarpus produced with no Quadrol treatment. A and C, transmitted, and B and D, polarized light microscopic images. Panels C and D are magnified images in the frames on panels A and B, respectively. The directions of bone are above, anterior; right, lateral; left, medial; and bottom, posterior. The field widths equal 3.57 mm (A, B) and 2.15 mm (C, D).
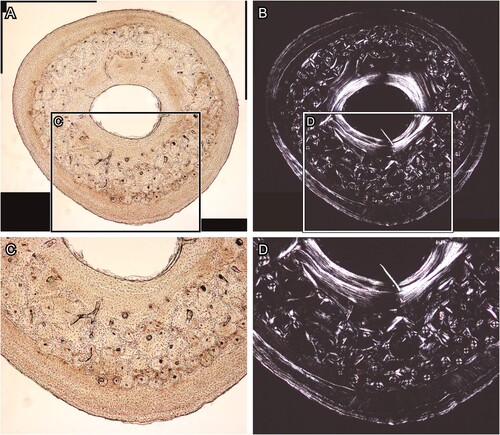
FIGURE 4. Transverse section of chicken (Gallus gallus) ulna, which was polished by silicon carbide and alumina abrasive powder. A and C, the BSE compositional images, and B and D, the BSE topographic images taken in the same area in the previous panels. Panels C and D are magnified images in the frames on panels A and B, respectively. Abbreviations: ab, abrasive powder; and ca, canal. The field widths equal 1.59 mm (A, B) and 706 μm (C, D).
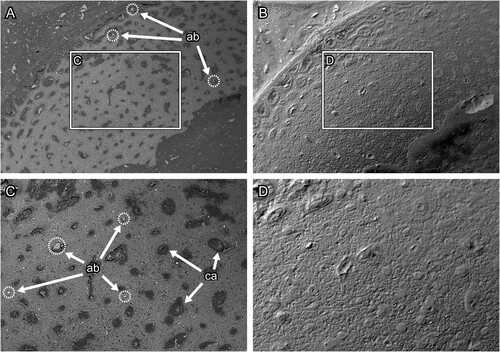
TABLE 1. List of materials, chemicals, and instruments used for the modified thin-sectioning method proposed in this study. See Figure 5 for the procedure of the modified thin sectioning method.
FIGURE 5. Diagram of a modified method of thin sectioning preparation developed in this study. The dashed frames and arrows indicate the procedures if a bone sample is required to be embedded in epoxy resin.
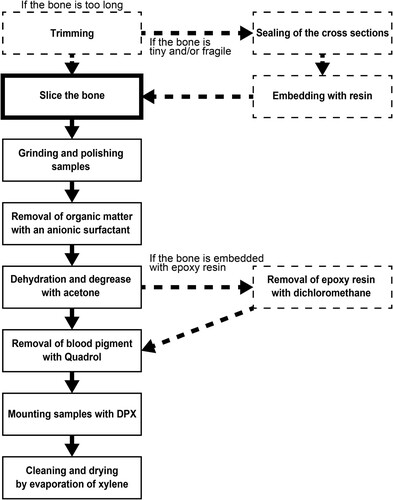
FIGURE 6. Transverse thin section of lesser Japanese mole (Mogera imaizumii) left humerus prepared by the method developed in this study (see the heading Modified Thin Sectioning Method). A, C, E, transmitted and B, D, F, polarized light microscopic images. The directions of bone are above, anterior; right, medial; left, lateral; and bottom, posterior. Abbreviation: ca, canal. The field widths equal 4.18 mm (A, B), 2.15 mm (C, D), and 845 μm (E, F).
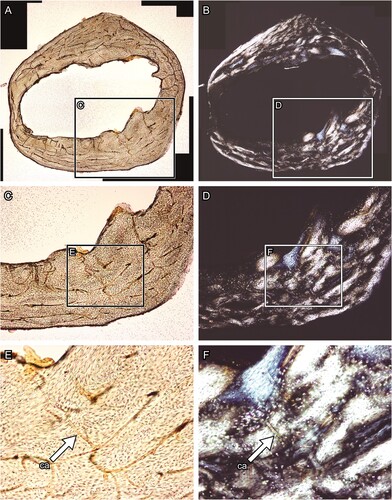
FIGURE 7. A, Chicken (Gallus gallus) humerus embedded by epoxy resin (the block), which is used for removal of epoxy resin (see the heading Removal of Epoxy Resin from the Samples). The block has been soaked in acetone and dichloromethane for: B, one; C, two; and D, three cycles. All scale bars equal 3 cm.
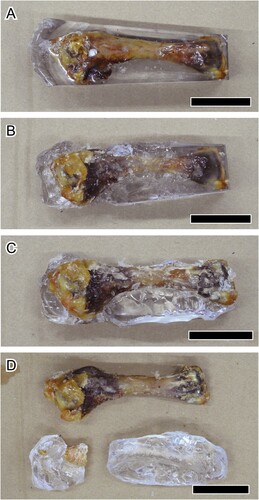
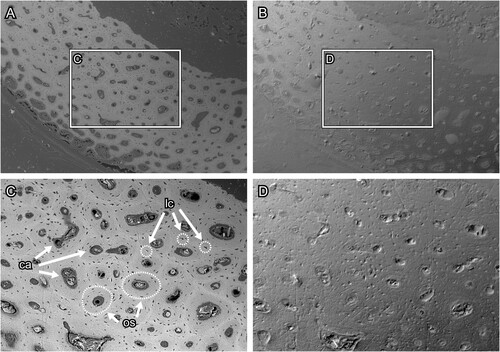
FIGURE 8. Transverse section surface of chicken (Gallus gallus) ulna, which was polished by silicon carbide paper. A and C, the BSE compositional images and B and D, the BSE topographic images taken in the same area in the previous panels. Figures C and D are magnified images in the frames on figures A and B, respectively. Abbreviations: ca, canal; lc, lacuna; and os, osteon. The field widths equal 1.59 mm (A, B) and 706 μm (C, D).
Supplemental Material
Download MS Word (1.8 MB)DATA AVAILABILITY STATEMENT
The high-resolution images of thin sections used in this study are available on Morphobank: http://morphobank.org/permalink/?P4522.
