Figures & data
Figure 1. Study visits. *Mometasone furoate inhalation powder 220 mcg, 1 inhalation twice daily, to be used during screening and washout periods. ICS, inhaled corticosteroid.
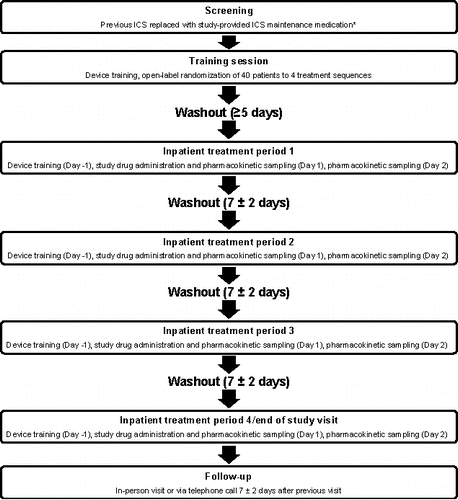
Figure 2. Patient disposition. *Of the 43 enrolled patients, 3 patients withdrew consent before receiving any study drug. FAS, full analysis set; Fp DPI, fluticasone propionate dry powder inhaler; Fp MDPI, fluticasone propionate multidose dry powder inhaler; FS DPI, fluticasone propionate/salmeterol dry powder inhaler; FS MDPI, fluticasone propionate/salmeterol multidose dry powder inhaler; PK, pharmacokinetic analysis set.
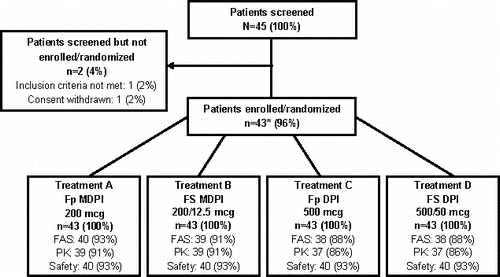
Table 1. Patient demographics and baseline characteristics, intent-to-treat population.
Figure 3. Plasma concentration-vs-time profiles for fluticasone propionate (a) and salmeterol (b), pharmacokinetic analysis set. Fp DPI, fluticasone propionate dry powder inhaler 500 mcg; Fp MDPI, fluticasone propionate multidose dry powder inhaler 200 mcg; FS DPI, fluticasone propionate/salmeterol dry powder inhaler 500/50 mcg; FS MDPI, fluticasone propionate/salmeterol multidose dry powder inhaler 200/12.5 mcg.
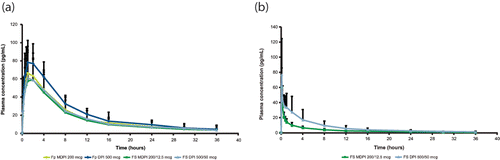
Table 2. Fluticasone propionate plasma pharmacokinetic parameters, pharmacokinetic analysis set.
Table 3. Fluticasone propionate treatment comparisons, pharmacokinetic analysis set.
Table 4. Salmeterol plasma pharmacokinetic parameters, pharmacokinetic analysis set.
Table 5. Salmeterol treatment comparisons, pharmacokinetic analysis set.
