Figures & data
Figure 1. Study disposition. Abbreviations: BID, twice daily; FF, fluticasone furoate; FP, fluticasone propionate; ITT, intent-to-treat; OD, once daily; PP, per protocol; SAL, salmeterol; VI, vilanterol.
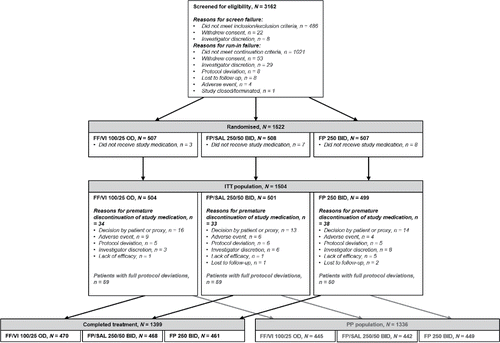
Table 1. Patient demographics and baseline characteristics (ITT population).
Table 2. Statistical analysis of change from baseline in evening trough FEV1 (L) at Week 24a.
Figure 2. Change from baseline in evening trough FEV1 in (a) the ITT population and (b) the PP population (repeated measures analysis). (a) ITT population. (b) PP population. Abbreviations: BID, twice daily; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; FP, fluticasone propionate; ITT, intent-to-treat; LS, least squares; OD, once daily; PP, per protocol; SAL, salmeterol; VI, vilanterol.
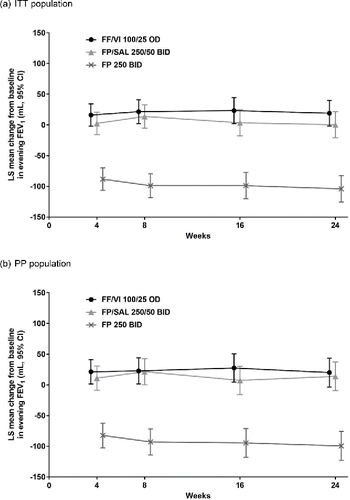
Figure 3. Treatment effect for primary/secondary/other efficacy endpoints (ITT analysis). (a) FF/VI 100/25 OD versus FP/SAL 250/50 BID. (b) FF/VI 100/25 OD versus FP 250 BID. (c) FP/SAL 250/50 BID versus FP 250 BID. Abbreviations: ACT, Asthma Control Test; AQLQ (12+), Asthma Quality of Life Questionnaire for 12 years and older; AM, morning; BID, twice daily; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FF, fluticasone furoate; FP, fluticasone propionate; OD, once daily; PEF, peak expiratory flow; PM, evening; SAL, salmeterol; VI, vilanterol.
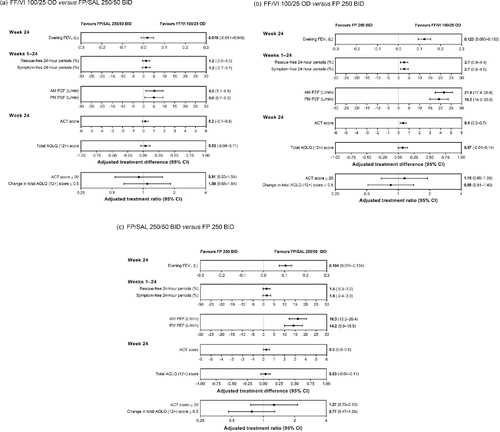
Table 3. Summary of AEs, SAEs and AEs of special interest (ITT population; N = 1504).
