Figures & data
Figure 1. Patient flow through study.
Notes. ACT: Asthma Control Test; AE: adverse event; AQLQ: Asthma Quality of Life Questionnaire; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; PD: protocol deviation; WPAI: asthma: Work Productivity and Activity Impairment: Asthma questionnaire. aThe total population includes all randomly assigned patients who received at least one prescription of study medication, were included in the ICS/LABA stratum and whose prerandomisation intended prescription was FP/Salm. bWeek 24 AQLQ data are not reported for this FP/Salm subset analysis. Other endpoints, including safety, exacerbations and prescribed/dispensed/collected medications, were captured throughout the study.
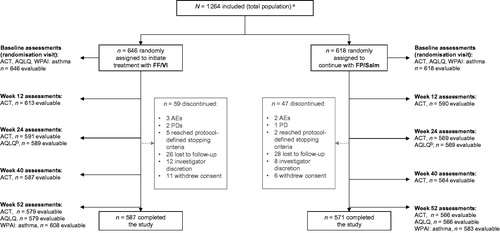
Table 1. Patient demographics and baseline asthma characteristics (total population).
Figure 2. ACT responders over time in the FF/VI and FP/Salm groups (total population).a.b
Notes. ACT: Asthma Control Test; CI: confidence interval; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; OR: odds ratio. aResponders defined as patients who achieved an ACT total score of ≥20 and/or improvement from baseline of ≥3 points. bLogistic regression analysis adjusting for randomised treatment, baseline ACT total score, baseline ACT total score squared, gender, and age.
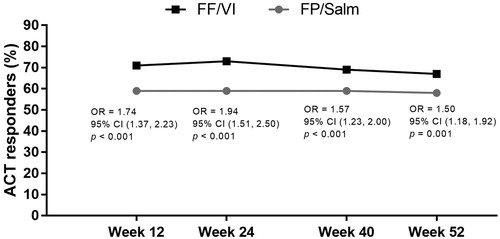
Figure 3. LS mean change from baseline in ACT total score over time in the FF/VI and FP/Salm groups (total population).a
Notes. ACT: Asthma Control Test; CI: confidence interval; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; LS: least squares; SE: standard error. aMixed model repeated measures analysis adjusted for randomised treatment, baseline ACT total score, randomised treatment-by-baseline ACT total score interaction, gender, age, visit and randomised treatment-by-visit interaction with an unstructured covariance matrix.
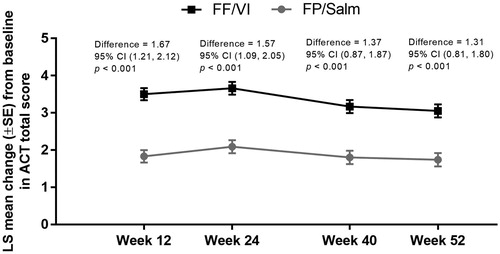
Figure 4. Percentage of responders for AQLQ total score and individual AQLQ domains in the FF/VI and FP/Salm groups (total population).a,b
Notes. AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FF/VI: fluticasone furoate/vilanterol; FP/Salm: fluticasone propionate/salmeterol; OR: odds ratio. aResponders defined as patients with a change from baseline of ≥0.5 points in AQLQ total score or AQLQ domain score. bLogistic regression analysis adjusting for randomised treatment, ACT total score at baseline per randomisation stratum, gender, age and baseline AQLQ score.
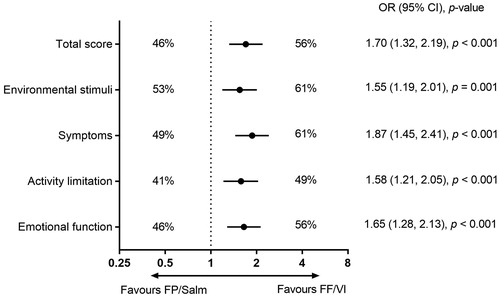
Table 2. On-treatment SAEs of special interest (total population).
