Figures & data
Figure 1. Summary of patient disposition (AP population). *The electronic case report form did not allow a reason for prescreen failure to be defined. †Including protocol-defined continuation criteria. Prescreen failure includes any patient who was assigned a patient number but did not complete any screening procedures. Screen failure includes any patient who completed at least one screening procedure, but the patient/caregiver did not attempt to self-administer a dose of mepolizumab via an AI. Study completion includes any patient who completed all assessments at the end of study visit. Two patients were withdrawn from the study after self-administering their first dose of mepolizumab; one patient owing to serious AEs relating to a traffic accident and one patient owing to physician decision (failure to comply with study procedures and unreliability). AE: adverse event; AI: autoinjector; AP: all patients enrolled.
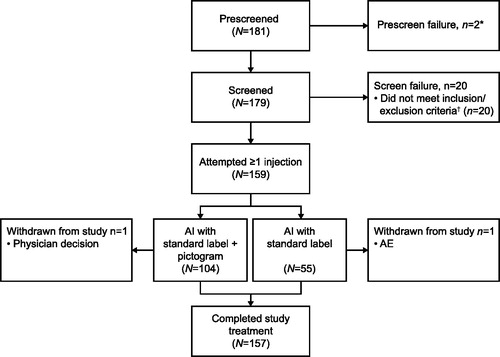
Table 1. Demographics and baseline characteristics (APT population).
Table 2. Summary of injection success including observer and at-home checklists (APT population).
Table 3. Patient/caregiver perception of AI usability (APT population).
Figure 2. Mean plasma mepolizumab concentration-time plots (Ctrough) by baseline mepolizumab use (APT population). Data are presented as mean ± SD. Lower limit of quantification was 50 ng/mL. APT: all patients treated; SD: standard deviation.
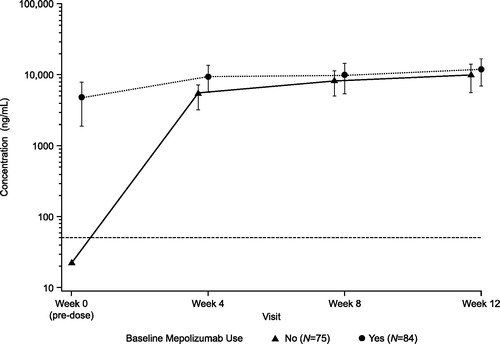
Figure 3. Absolute blood eosinophils by baseline mepolizumab use (PD population). Data are presented as geometric mean ± 95% CI. CI: confidence interval; PD: pharmacodynamic.
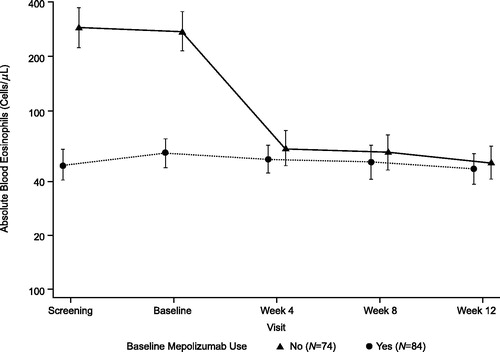
Table 4. Adverse events (APT population).
Table 5. Injection pain summary (APT population).
Supplemental Material
Download MS Word (199.8 KB)Data sharing statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
