Figures & data
Table 1. Baseline characteristics by asthma severity and eosinophil status.
Figure 1. HCRU per patient year by (A) asthma severity* and (B) blood eosinophil count†. *Patients were divided into two groups according to time spent in the non-severe and severe categories. †Blood eosinophil count categories: <300 cells/μL (throughout the follow-up period); ≥300 cells/μL (at any time during the follow-up period); unknown (no blood eosinophil count available). Severe eosinophilic asthma was defined as severe asthma with a blood eosinophil count of ≥300 cells/μL. ‡Outpatient visits include scheduled and ER outpatient visits and scheduled telephone calls from which data were recorded in the patient files. BEC: blood eosinophil count; HCRU: health care resource utilization; ER: emergency room.
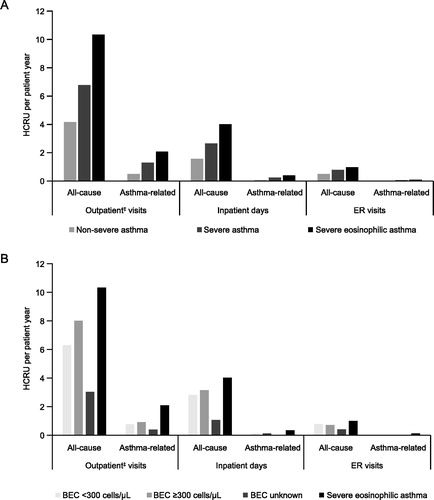
Figure 2. Annual HCRU from index date to Year 5 of follow-up in patients according to asthma severity. *Patients were divided into two groups according to time spent in the non-severe and severe categories. HCRU: health care resource utilization.
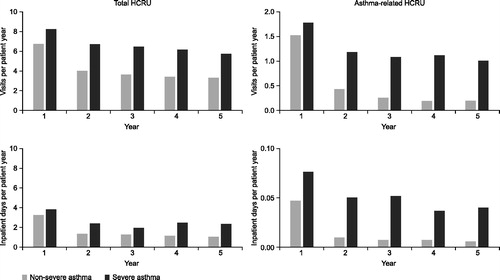
Table 2. HCRU-Associated total and asthma-related costs by asthma severity and blood eosinophil count.
Figure 3. Overall survival by (A) asthma severity* and (B) blood eosinophil count† and competing risk models for non-asthma and asthma-related mortality by (C) asthma severity* and (D) blood eosinophil count†. *Patients were divided into two groups by baseline disease severity status: non-severe and severe. †Blood eosinophil count categories: <300 cells/μL (throughout the follow-up period); ≥300 cells/μL (at any time during the follow-up period); unknown (no blood eosinophil count available). BEC: blood eosinophil count.
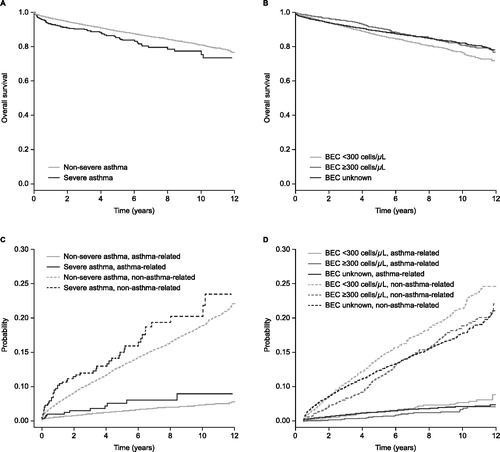
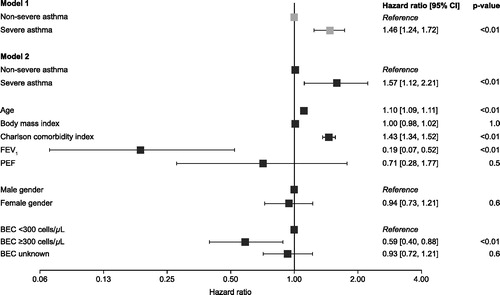
Figure 4. Cox regression models of all-cause mortality by disease severity* and patient characteristics. *To account for patients with transitioning asthma (non-severe asthma at index but presented with severe asthma during follow‐up), severity was modeled as a time-varying covariate. CI: confidence interval; BEC: blood eosinophil count; FEV1: forced expiratory volume in 1 s; PEF: peak expiratory flow.
Supplemental Material
Download MS Word (19.7 KB)Data availability statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The sharing of a de-identified dataset of this study is restricted by Finnish law (Data Protection Act (1050/2018)). The dataset can only be requested through permit authorization process from Turku Clinical Research Center for justifiable research projects (http://www.turkucrc.fi/en).
