Figures & data
Figure 1. Study design. aIncluded patients who switched to another ICS/LABA FDC or to a different dose of the index ICS/LABA. B/F, budesonide/formoterol; FF/VI, fluticasone furoate/vilanterol; FDC, fixed-dose combination; FP/SAL, fluticasone propionate; ICS/LABA, inhaled corticosteroid/long-acting β2-agonist.
Figure 2. Study population selection. aDuring the baseline or follow-up period. bDuring the baseline period or on the index date. B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FDC, fixed-dose combination; FF/VI, fluticasone furoate/vilanterol; FP/SAL, fluticasone propionate; ICS/LABA, inhaled corticosteroid/long-acting β2-agonist.
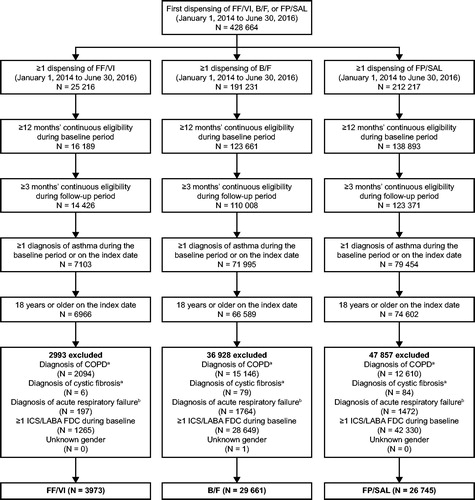
Table 1. Selected post-match baseline characteristics.
Figure 3. Mean PDC (A) and proportion of patients achieving PDC ≥ 0.5 and PDC ≥ 0.8 with FF/VI versus B/F (B) and FF/VI versus FP/SAL (C). Mean PDC and OR were calculated using generalized linear regression and logistic regression models, respectively, including the following baseline covariates: (A) FF/VI versus B/F: region, insurance product type, log of all-cause medical cost, log of all-cause medical cost paid by patient, log of all-cause outpatient costs, number of all-cause outpatient visits, and baseline LAMA/LABA use (yes/no); (B) FF/VI versus FP/SAL: insurance product type, log of all-cause total cost, log of all-cause medical cost, log of all-cause ED visit cost, log of all-cause outpatient costs, log of all-cause medical cost paid by patient, number of all-cause ED visits, number of all-cause outpatient visits, and baseline rescue medication use (yes/no). B/F, budesonide/formoterol; CI, confidence interval; ED, emergency department; FF/VI, fluticasone furoate/vilanterol; FP/SAL, fluticasone propionate; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; OR, odds ratio; PDC, proportion of days covered.
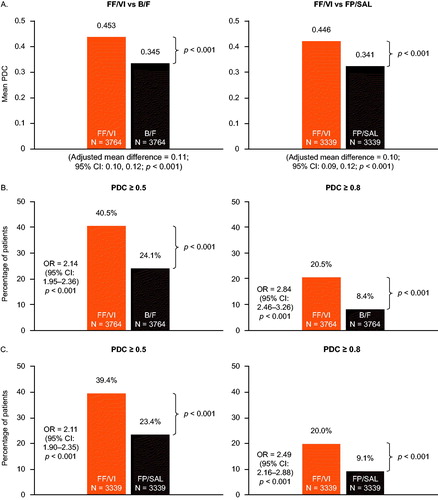
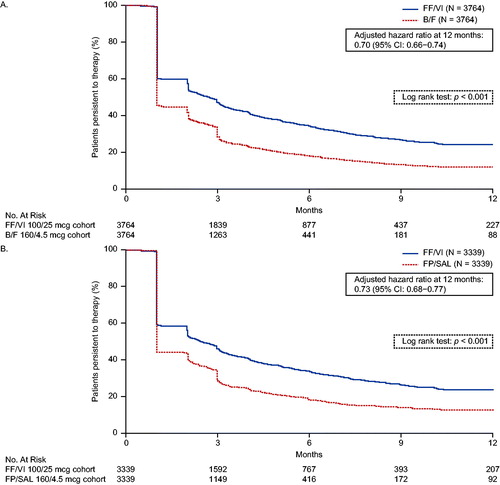
Figure 4. Kaplan–Meier analysis of persistence to (A) FF/VI versus B/F and (B) FF/VI versus FP/SAL. Treatment discontinuation was defined as a gap in therapy of >45 days from the run-out date of the ICS/LABA pharmacy fill and the next fill, or between the end of the days of supply of the last dispensing and the end of follow-up, whichever occurred first. Hazard ratios were calculated using a Cox proportional hazards model adjusted for baseline covariates including: (A) FF/VI versus B/F: region, insurance product type, log of all-cause medical cost, log of all-cause medical cost paid by patient, log of all-cause outpatient costs, number of all-cause outpatient visits, and baseline LAMA/LABA use (yes/no); (B) FF/VI versus FP/SAL: insurance product type, log of all-cause total cost, log of all-cause medical cost, log of all-cause ED cost, log of all-cause outpatient costs, log of all-cause medical cost paid by patient, number of all-cause ED visits, number of all-cause outpatient visits, and baseline rescue medication use (yes/no). B/F, budesonide/formoterol; ED, emergency department; FF/VI, fluticasone furoate/vilanterol; FP/SAL, fluticasone propionate; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Supplemental Material
Download (139.6 KB)Data sharing
To request access to patient-level data and documents for this study, please submit an enquiry via www.clinicalstudydatarequest.com. Data included in this manuscript are derived from a database owned by IQVIATM and contain proprietary elements, and therefore data cannot be broadly disclosed or made publicly available at this time. The disclosure of this data to third-party clients assumes certain data security and privacy protocols are in place and that the third-party client has executed IQVIA’s standard license agreement, which includes restrictive covenants governing the use of the data.
