Figures & data
Figure 1. Study design scheme for (A) the asthma cohort and (B) the treatment cohorts. HRU, healthcare resource utilization; ICS: inhaled corticosteroid; LABA: long-acting beta2 agonist.
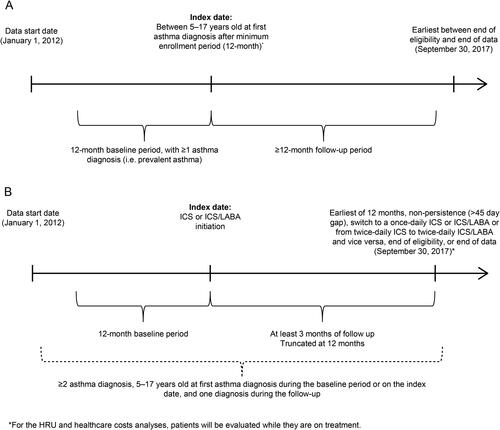
Table 1. Baseline characteristics: asthma cohort.a
Table 2. Follow-up treatment patterns: asthma cohort.
Table 3. Follow-up asthma-related HRU and healthcare costs: asthma cohort and treatment cohort.
Table 4. Baseline characteristics: treatment cohorts.
Table 5. Follow-up treatment patterns: treatment cohorts.
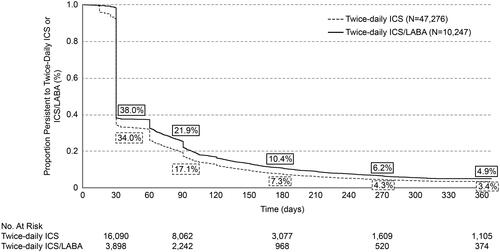
Figure 2. Kaplan-Meier rates of persistencea to twice-daily ICS or ICS/LABA. Notes: aNon-persistence (i.e. discontinuation) was defined as a gap of >45 days in days’ supply of medication (i.e. between the end of supply of one dispensing to the date of the following dispensing or the end of the follow-up period). ICS: inhaled corticosteroid; LABA: long-acting beta2 agonist.
208274_Supp_Fig_2_V2.pdf
Download PDF (123.3 KB)208274_Supp_Fig_1_V2.pdf
Download PDF (124.9 KB)208274_MS_Supplementary_Material.docx
Download MS Word (501.4 KB)Data sharing statement
To request access to de-identified patient-level data and documents for this study, please submit an enquiry via www.clinicalstudydatarequest.com. Data included in this manuscript are derived from a database owned by IQVIA and contain proprietary elements, and therefore data cannot be broadly disclosed or made publicly available at this time. The disclosure of this data to third-party clients assumes certain data security and privacy protocols are in place and that the third-party client has executed IQVIA’s standard license agreement, which includes restrictive covenants governing the use of the data.
