Figures & data
Table 1. Model cohort characteristics at model start.†
Figure 1. Model structure.
Color coding: Black: Absorbing health state; Dark gray: Treatment-related health state; White: Sub-states within treatment-related health states.
*Add-on treatment may be any one of the following monoclonal antibodies: dupilumab, mepolizumab, benralizumab.
†Moderate exacerbations are defined based on the loss of asthma control (LOAC) events (excluding severe exacerbation events) as collected in the QUEST trial. As such, at least one of the following criteria must be satisfied to count as a moderate exacerbation: ≥6 additional reliever puffs of salbutamol/albuterol or levosalbutamol/levalbuterol in a 24-hour period (compared with the baseline) on two consecutive days; ≥20% decrease in pre-bronchodilator forced expiratory volume in one second (FEV1) compared with the baseline; Increase in inhaled corticosteroid (ICS) dose ≥4 times than the dose at Visit 2; A decrease in AM or PM peak flow of 30% or more on two consecutive days of treatment, based on the defined stability limit. The treatment period stability limit is defined as the respective mean AM or PM peak expiratory flow (PEF) obtained over the last seven days prior to randomization (Day 1). ΔSevere exacerbations are defined based on the severe exacerbation events as collected in the dupilumab trials. As such, at least one of the following criteria must be satisfied to count as a severe exacerbation: Use of systemic corticosteroids for ≥3 days; Hospitalization or emergency room visit because of asthma, requiring systemic corticosteroids.
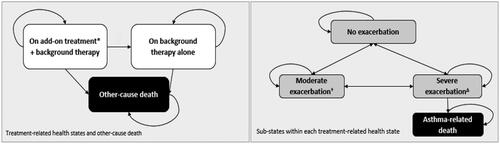
Table 2. Overview of the main cost inputs.
Table 3. Base case results for the benralizumab like population.
Table 4. Base case results for the mepolizumab like population.
Suppl_Tables1-9_and_Suppl_Figures1-4__v.Final_20210609_STC.docx
Download MS Word (839 KB)Data availability statement
All data supporting the findings of this study are available within the article and its supplementary materials.
