Figures & data
Figure 1. Mean (SD) number of exacerbations in the year prior to study baseline and unadjusted annualized severe exacerbation rate over treatment periods: type 2 patients enrolled from (A) QUEST and (B) Phase 2b, with or without evidence of an allergic phenotype and non–type 2 patients with evidence of an allergic phenotype at parent study baseline. SD, standard deviation.

Figure 2. Mean change from parent study baseline over the treatment period in pre-bronchodilator FEV1: type 2 patients enrolled from (A) QUEST and (B) Phase 2b, with or without evidence of allergic asthma and non–type 2 patients with evidence of allergic asthma at parent study baseline. DPL, dupilumab; FEV1, forced expiratory volume in 1 s; PBO, placebo; SE, standard error.

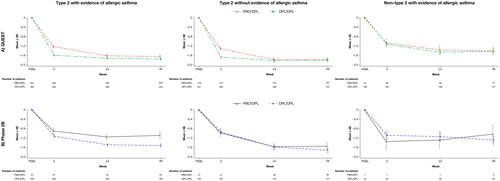
Figure 3. Mean change from parent study baseline over the treatment period in ACQ-5 score: type 2 patients enrolled from (A) QUEST and (B) Phase 2b, with or without evidence of allergic asthma and non–type 2 patients with evidence of allergic asthma at parent study baseline. ACQ-5, 5-item Asthma Control Questionnaire; DPL, dupilumab; PBO, placebo; SE, standard error.
Supplemental Material
Download MS Word (1.9 MB)Supplemental Material
Download PDF (94.1 KB)Supplemental Material
Download PDF (108.4 KB)Supplemental Material
Download PDF (88 KB)Supplemental Material
Download PDF (125.6 KB)Supplemental Material
Download PDF (111.7 KB)Supplemental Material
Download PDF (177.7 KB)Supplemental Material
Download PDF (141.6 KB)Supplemental Material
Download PDF (149.9 KB)Supplemental Material
Download PDF (150.2 KB)Data availability statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org
