Figures & data
Figure 1. Patient disposition. BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
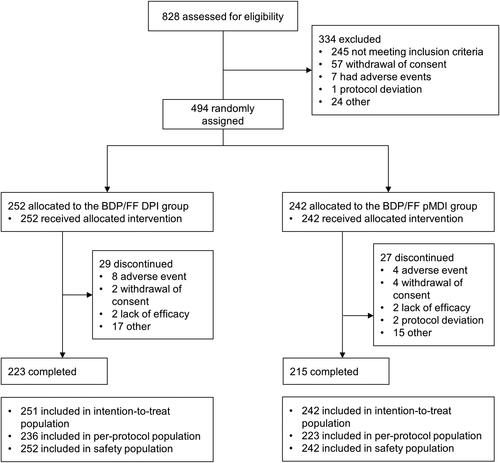
Table 1. Demographics and disease characteristics at screening (ITT population).
Figure 2. Pre-dose morning PEF (ITT population). *p<.05 DPI vs. pMDI. Data are adjusted mean and 95% confidence interval. Entire treatment period analyzed using analysis of covariance; individual periods analyzed using mixed model for repeated measures. Mean baseline values were 383.6 and 384.1 L/min for BDP/FF DPI and pMDI, respectively. PEF: peak expiratory flow; BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
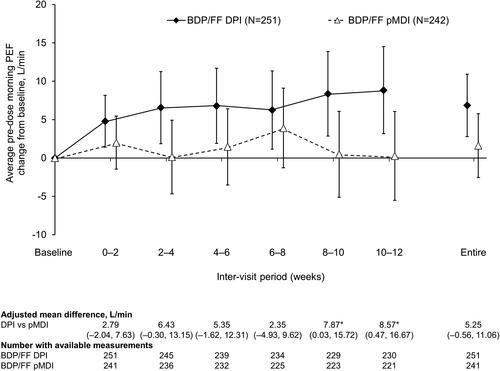
Figure 3. Pre-dose evening PEF (ITT population). *p<.05 DPI vs. pMDI. Data are adjusted mean and 95% confidence interval, analyzed using mixed model for repeated measures. Mean baseline values were 388.8 and 390.5 L/min for BDP/FF DPI and pMDI, respectively. PEF: peak expiratory flow; BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
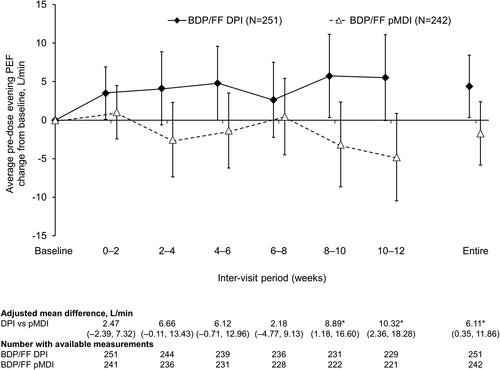
Figure 4. Percentage of asthma control days (ITT population). Data are adjusted mean and 95% confidence interval, analyzed using mixed model for repeated measures. Mean baseline values were 75.21 and 74.80% for BDP/FF DPI and pMDI, respectively. BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
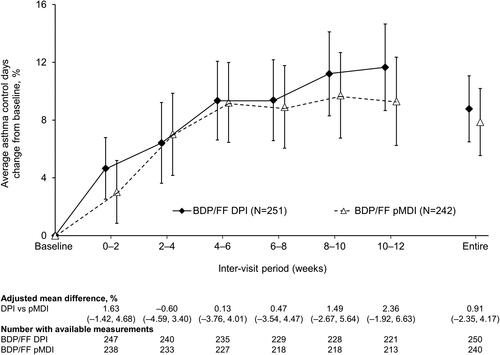
Figure 5. Pre-dose FEV1 (ITT population). Data are adjusted mean and 95% confidence interval, analyzed using mixed model for repeated measures. Mean baseline values were 2.81 and 2.85 L for BDP/FF DPI and pMDI, respectively. FEV1: forced expiratory volume in 1 s; BDP/FF: beclomethasone dipropionate/formoterol fumarate; DPI: dry-powder inhaler; pMDI: pressurized metered dose inhaler.
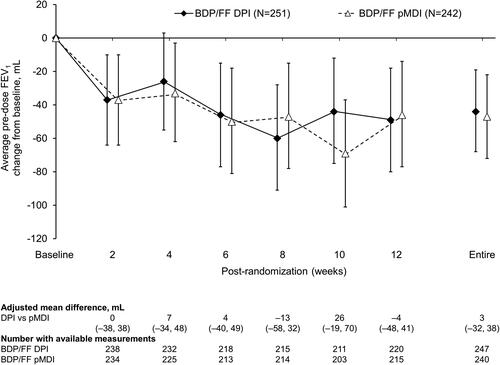
Table 2. Treatment-emergent adverse events and serious adverse events (safety population).
Supplemental Material
Download PDF (411.1 KB)Data availability statement
Chiesi commits to sharing with qualified scientific and medical researchers, conducting legitimate research, the anonymized patient-level and study-level data, the clinical protocol and the full clinical study report of Chiesi Farmaceutici SpA-sponsored interventional clinical trials in patients for medicines and indications approved by the European Medicines Agency and/or the US Food and Drug Administration after 1 January 2015, following the approval of any received research proposal and the signature of a Data Sharing Agreement. Chiesi provides access to clinical trial information consistently with the principle of safeguarding commercially confidential information and patient privacy. Other information on Chiesi’s data sharing commitment, access and research request’s approval process are available in the Clinical Trial Transparency section of http://www.chiesi.com/en/research-and-development/.
