Figures & data
TABLE 1 Dipole moment and total bonding energy (TBE) of gas-phase sulfuric acid and sulfuric acid hydrates from CitationAl Natsheh et al. (2003)
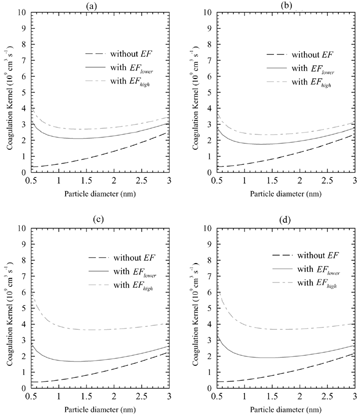
FIG. 1 The upper and lower limits of the ratio between the uptake coefficients calculated using the maximum and minimum values of the dipole moments from (EF upper , EF lower ) and those (EF ref ) calculated using the reference measured value of the dipole moment of sulfuric acid (2.73 D; Kuczkowski et al. 1981) for (a) free sulfuric acid molecules, (b) monohydrates, (c) dihydrates, and (d) trihydrates.
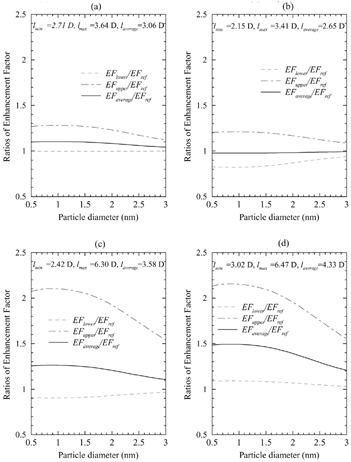
FIG. 2 The comparison of the uptake coefficients calculated using EquationEquation (2) with and without enhancement factors (EF) as a function of the particle diameter for (a) free sulfuric acid molecules, (b) monohydrates, (c) dihydrates, and (d) trihydrates at the ambient gas temperature of 300 K using the same input data set as that for .