Figures & data
TABLE 1 Summary of the instrumentation deployed during sampling in Egbert
FIG. 1 Time series of ammonium, nitrate, sulfate and organics mass concentrations of ambient aerosols measured with the AMS, together with ambient temperature (T), relative humidity (RH), wind speed (ws) and wind directions (wd) at CARE from March 27 to May 8, 2003. The chemical components are 15 min averages and the meteorological parameters are 10 min averages. The gas phase concentrations of ammonia from filter pack analysis (24 h averages) are also shown.
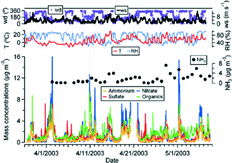
TABLE 2 Summary statistics of ambient mass concentrations (μg m−3) of chemical constituents in submicron aerosols, and aerosol acidity. The total mass is the sum of only ammonium, nitrate, sulfate and organics. Aerosol acidity is the molar ratio of ammonium to sum of two times sulfate and nitrate [NH4/(2SO4 + NO3)]. SD indicates the standard deviation
FIG. 2 Correlations among aerosol components measured with the Aerosol Mass Spectrometer (AMS). The acidity of the particles—the molar ratio of positive ions to negative ions (NH4 +/2SO4 = + NO3 −)—is also displayed (d).
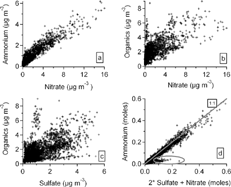
FIG. 3 The average mass size distributions of ammonium, nitrate, sulfate and organics in ambient aerosols acquired by the aerosol mass spectrometer for the entire sampling period.
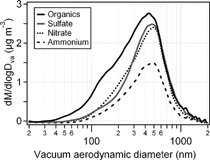
FIG. 4 The average mass size distribution of AMS total mass (sum of ammonium, nitrate, sulfate and organics) and mass size distribution estimated from number size distributions from SMPS and APS, assuming that particles are spherical with a particle density of 1.5 g cm−3. Estimated transmission efficiencies (TE) of the AMS inlet lens as a function of particle vacuum aerodynamic diameters are also shown.
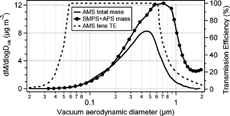
FIG. 5 Intercomparison among hourly AMS NR-PM1 (AMS total mass concentrations—the sum of only ammonium, nitrate, sulfate, and organics mass concentrations), SMPS + APS PM2.5 (estimated PM2.5 mass concentrations from number distributions measured with the SMPS and APS) and TEOM PM2.5 mass concentrations. The APS mass concentrations of particles with aerodynamic diameters 0.5–2.5 μm (APS < 2.5 μm) and the AMS derived nitrate mass concentrations are also shown.
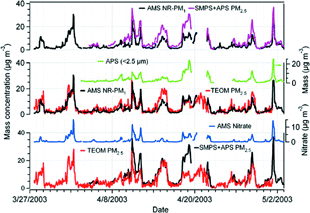
FIG. 6 Time series of particulate ammonium, nitrate and sulfate mass concentrations at Egbert measured with the AMS and those species from the filter pack analysis of PM2.5 (both data 24 h averages).
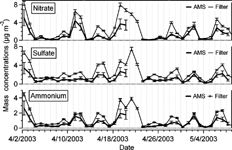
FIG. 7 Intercomparison of 24 h averaged particulate ammonium, nitrate and sulfate mass concentrations acquired by the AMS and filter measurements of PM2.5.
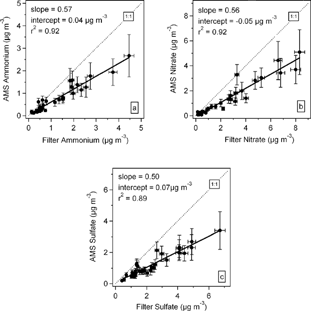
FIG. 8 Intercomparison between particulate nitrate measured by the AMS and the R&P8400N particulate nitrate monitor (both 1 h averages).
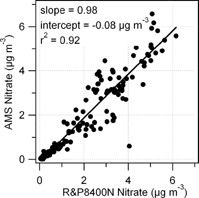
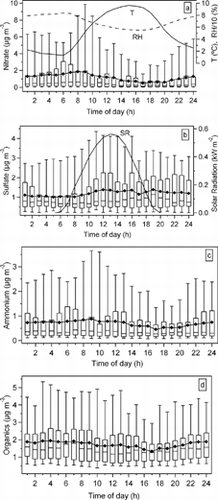
FIG. 9 Diurnal patterns of (a) nitrate, (b) sulfate, (c) ammonium, and (d) organics mass concentrations along with diurnal variations of ambient temperature (T), relative humidity (RH) and solar radiation (SR). In each box, the mid-line (−) represents the median value and a marker • represents the average for each hour. The top and bottom of the box represent the upper and lower quartiles respectively. The top and bottom of the whisker represent the 95th percentile and 5th percentile respectively.
Time series of total organics, organics contributing to m/z 57 (an indicator for hydrocarbon-like organic aerosol, HOA) and m/z 44 (an indicator for oxygenated organic aerosol, OOA) reported by the AMS, and gas phase mixing ratios of some selected non-methane hydrocarbons and ozone. EB indicates ethylbenzene and Org. indicates total organics.
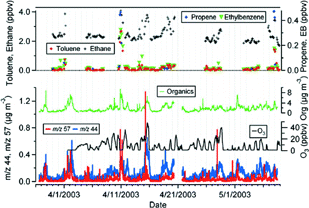
FIG. 11 Correlations between the mass concentrations of organics in small particles (< 200 nm) and mixing ratios of the gas phase non-methane hydrocarbon compounds. The correlations between those NMHCs and particulate m/z 57/organics and m/z 44/organics are also included.
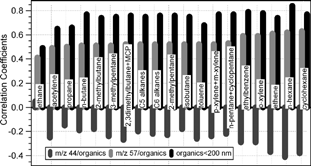
FIG. 12 Diurnal pattern of the (a) m/z 57/total organics—a fraction of the total particulate organics that represents hydrocarbon-like organics in the particles and (b) m/z 44/total organics—a fraction that represents oxygenated organics for the entire sampling period. The lengths of the box and whiskers are defined as in .
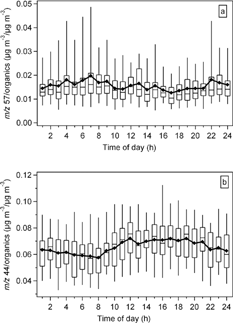
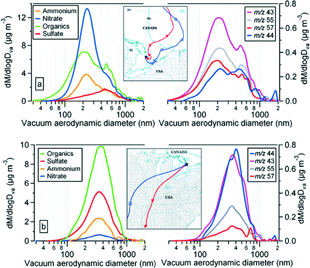
FIG. 13 Mass size distributions of particulate ammonium, nitrate, sulfate and organics during the two different events on (a) April 10–11 and (b) April 15 as described in the text. Mass distributions of selected key organic mass fragments are also included. Typical air mass back trajectories (red: 250 m and blue: 1000 m, and two marks along trajectory represent 24 h interval) for these events are also displayed.