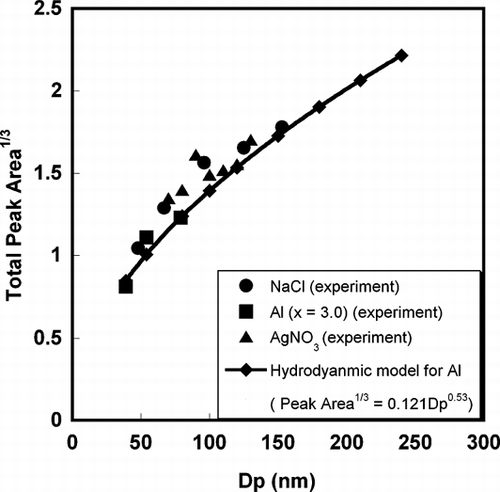
Figures & data
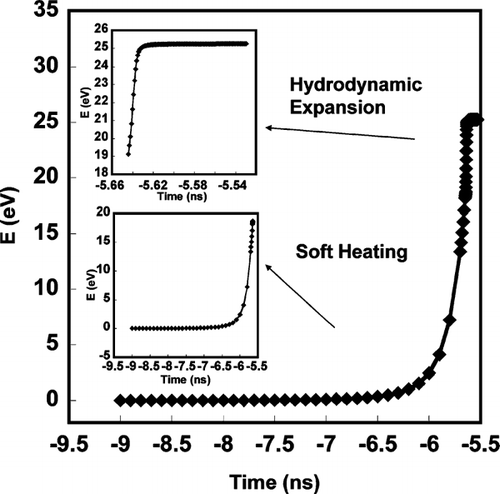
FIG. 1 Simulation of soft laser heating of 100 nm Aluminum as a function to time. Time = 0 corresponds to the peak in the 532 nm, 5 ns FWHM 100 mJ Gaussian laser pulse.
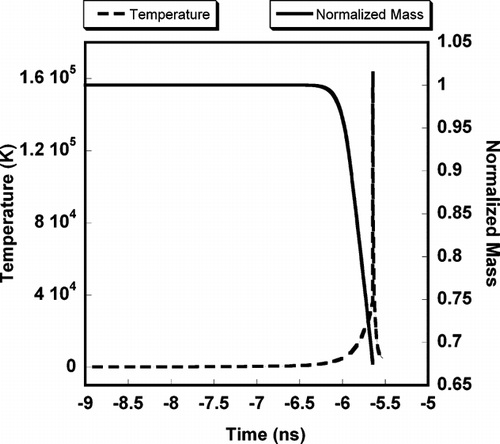
FIG. 2 (a) Temporal and radial spatial variation of normalized electron density. (b) Temporal and radial spatial variation of the normalized electric field.
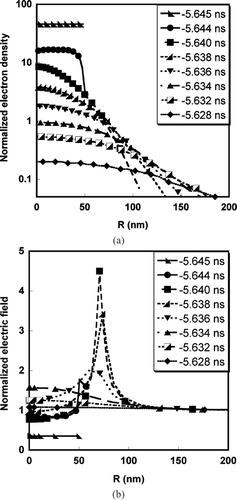
FIG. 5 (a) Kinetic energy profile as a function of radial distance at various times for an aluminum particle of initial diameter = 100 nm. (b) Temporal variation of the average and maximum kinetic energy for an aluminum particle of initial diameter = 100 nm.
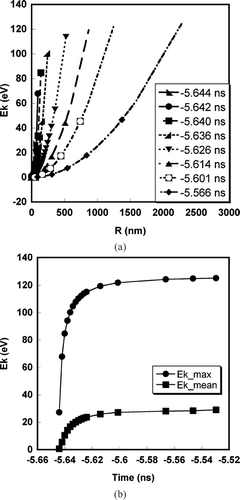
FIG. 6 Effect of laser pulse width on average kinetic energy and average ionization state for an aluminum particle of initial diameter = 100 nm.
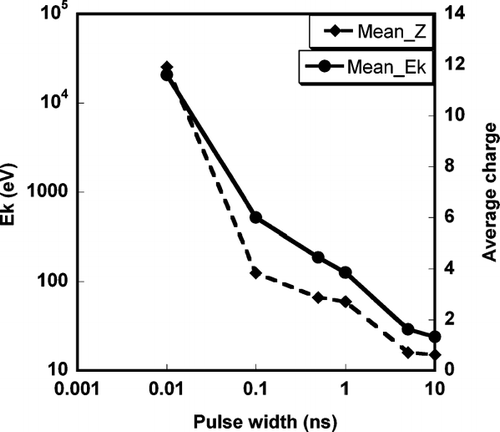
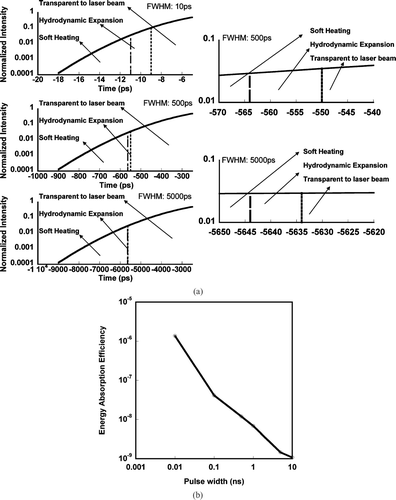
FIG. 7 (a) Left: Gaussian laser pulses of various width overlayed with the “soft-heating” and hydrodynamic expansion regions for an aluminum particle of initial diameter = 100 nm. Right: Detailed view of “soft-heating” and hydrodynamic expansion regions for FWHM 5000 ps and FWHM 500 ps laser pulses. (b) Calculated fraction of laser energy absorbed in a 100 nm aluminum particle as a function of pulse width.