Figures & data
FIG. 1 The DRH for NaCl particles determined for a range of sizes between 5 and 150 nm. (1) The dotted line is calculated using surface tensions within the experimental range (CitationRussell and Ming 2002). (2,3) DRH calculated using upper bounds of surface tension (CitationBahadur et al. 2007) for σSL = 59 mNm−1 and for σLV = 89 mNm−1. The solid line (2) is calculated using bulk values and the dashed line (3) includes Tolman corrections to the surface tension. (4,5) DRH calculated using solid-liquid surface tension calculated in this work for σSL = 59 mNm−1 and measured liquid-vapor surface tension of σLV = 80 mNm−1. The solid line (4) uses bulk values and dashed line (5) includes Tolman corrections. (6) Solid circles show measured NaCl DRH for nanoparticles (CitationBiskos et al. 2006).
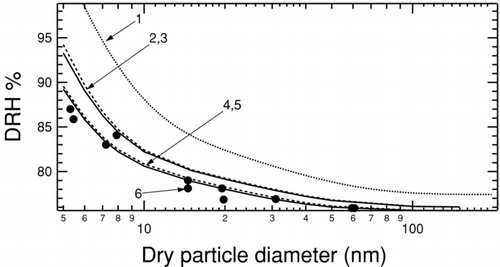
FIG. 2 Frequency of particle occurrence as a function of radial distance from the average particle center for an example NaCl particle with 3.5 nm edge length. For clarity polynomial fits are shown in this figure. The tail-end peaks are artifacts of the fit, and not a real part of the distribution. Part (a) shows the radial distribution in the test-area method for the reference (solid line) state, and the perturbed (dashed lines) states. Part (b) shows the radial distribution of the reference state at 1, 2, 3, 4, and 5 ns. The lines are colinear.
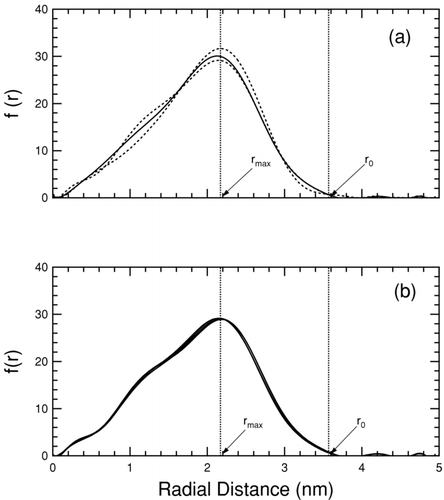
TABLE 1 Simulation cell edge length and number of included molecules
TABLE 2 Calculated surface tensions in NaCl-Air-water system
FIG. 3 Variation in water–air surface tension as a function of water particle diameter. Solid squares are surface tension upper bounds from the energy difference method, solid circles are surface tensions from the test-area method (both from this work), and empty circles correspond to measurements (CitationWingrave et al. 1981). The solid curved lines correspond to a two-parameter fit using Equation (Equation3) allowing both σ0 and δ to vary. Associated dashed lines are single-parameter fits obtained by holding σ0 constant at the calculated values listed in . All values of surface tension and Tolman length are summarized in .
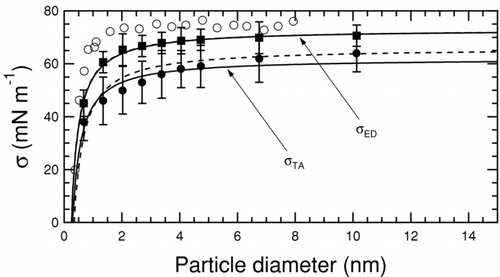
TABLE 3 Surface tensions and Tolman lengths from one (OP) and two (TP) parameter fits.
FIG. 4 Variation in surface tension as a function of particle size. The interface in each case exists between a particle of the more condensed phase embedded in the more diffuse phase. Solid squares show surface tension upper bounds from the energy difference method and solid circles are surface tensions from the test-area method. Solid lines correspond to a two parameter (TP) fit used to determine Tolman length, and dashed lines are a one parameter (OP) fit.
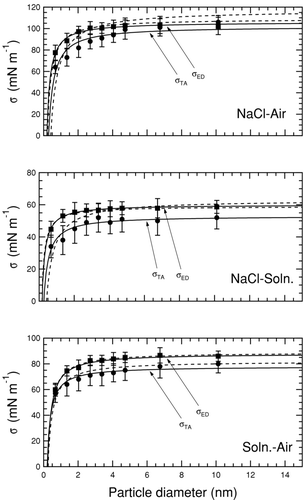
FIG. 5 Deliquescence relative humidity curves for NaCl at 300 K and 1 atm calculated using a bulk thermodynamic model (CitationRussell and Ming 2002). The solid–liquid surface tension is held constant at 63 mNm−1 and liquid-vapor surface tension is varied as indicated by tags.
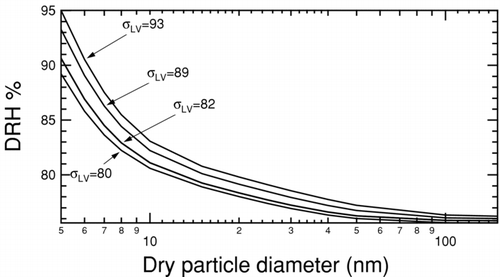
FIG. 6 Deliquescence relative humidity curves for NaCl at 300K and 1 atm calculated using a bulk thermodynamic model (CitationRussell and Ming 2002). The liquid–vapor surface tension is held constant at 82 mNm−1 and solid–liquid surface tension is varied as indicated by tags.
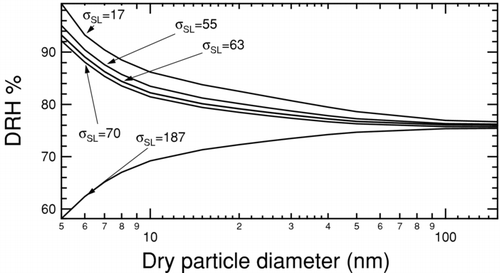
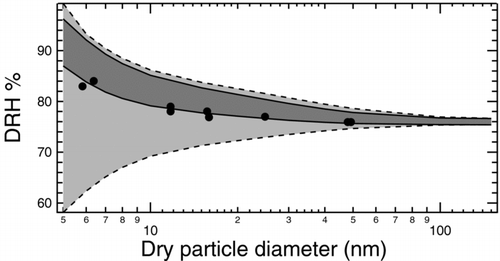
FIG. 7 Region of possible size-dependent NaCl DRH curves at 300 K and 1 atm due to uncertainty in surface tension. The light grey region delimited by dashed lines represents the area determined based on extreme reported values of surface tension. The upper limit uses 85 mNm−1 for σLV and 17 mNm−1 for σSL and the lower limit uses 80 mNm−1 and 187 mNm−1, respectively. The dark grey region delimited by solid lines represents the area determined by combining the reported value of 80 mNm−1 for σLV with the possible extreme values of 56 and 63 mNm−1 for σSL calculated in this work. Shape-corrected measurements of DRH (CitationBiskos et al. 2006) shown as filled circles lie close to or within the dark grey region, which is entirely a subset of the light grey region.