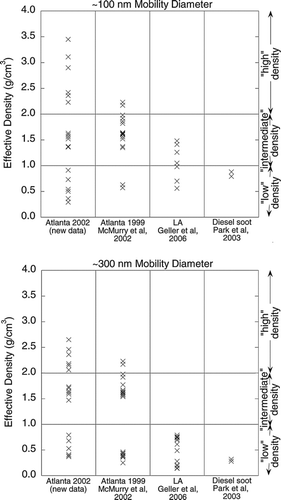
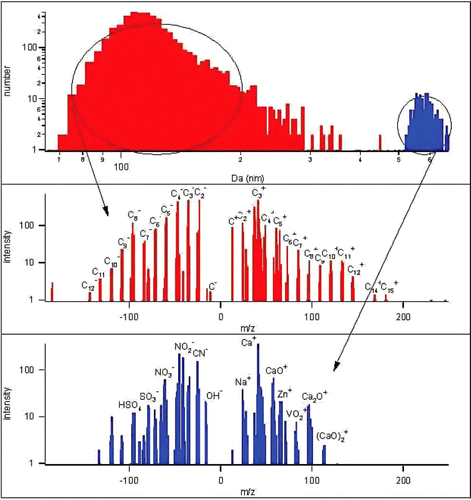
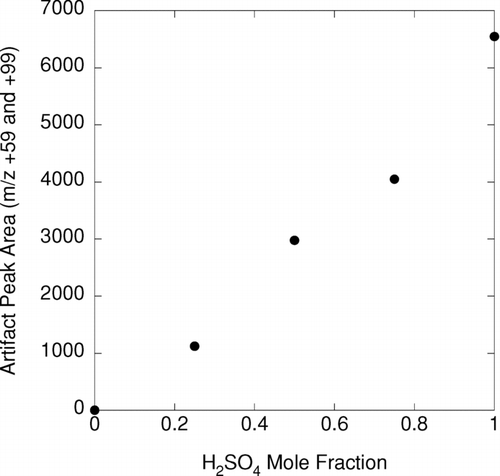
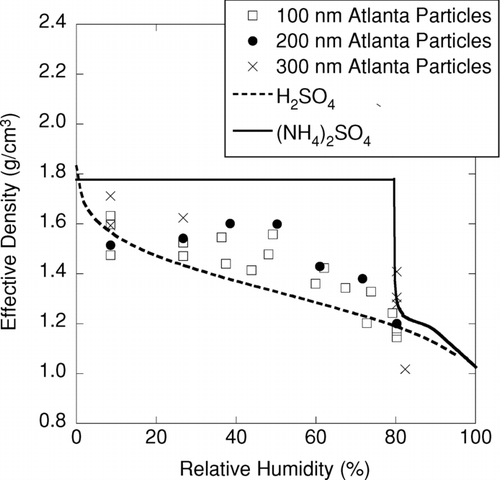
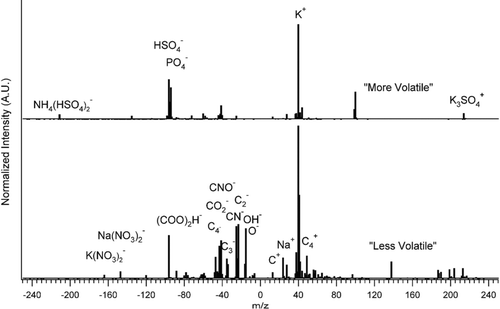
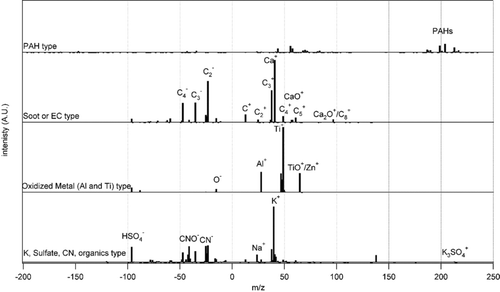
Figures & data
TABLE 3 Measurements of transport properties
TABLE 4 Measurements of particle physical/Chemical properties: DMA systems
TABLE 5 Measurements of particle physical/Chemical Properties: TDMA systems