Figures & data
FIG. 1 Particle-wall loss rates as a function of size for particles with 0 through 3 net charges (positive or negative) for a spherical chamber with a radius of 1 m and a turbulence factor, k e , of 1 s− 1 and a mean electric field magnitude of 50 volts cm− 1 (solid lines). Also shown is the time evolution of the net particle wall-loss rate (particle wall-loss rate summed over all charges) in the chamber when the particles initially have a Boltzmann charge distribution (dashed lines).
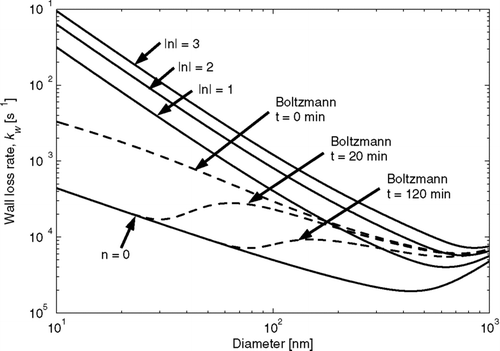
FIG. 2 Example of the modeled wall loss rates using the FLAT (1 free parameter: k w = 8 × 10− 5 s− 1), TURB (1 free parameter: k e = 1 s− 1), and HYBRID (2 free parameters: k w0 = 4 × 10− 5 s− 1, k e = 0.5 s− 1) assumptions.
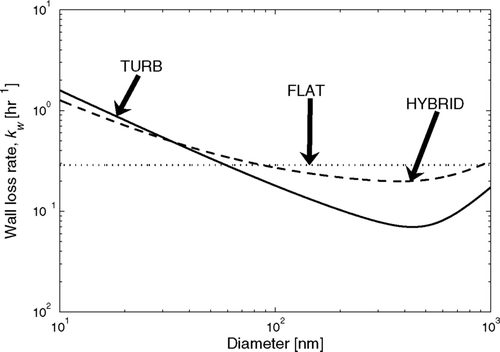
FIG. 3 Initial and final (4 h later) number and mass size distributions during the ammonium sulfate experiment.
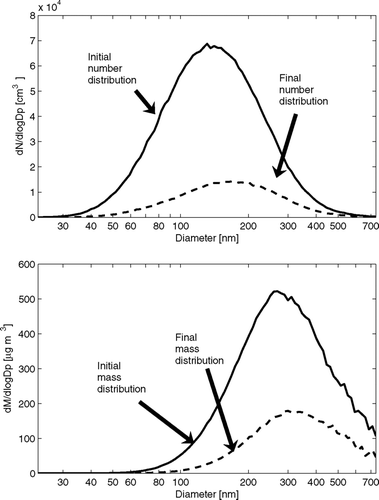
FIG. 4 The calculated total cumulative condensation onto the suspended particles as a function of time for the ammonium sulfate experiment. The predicted condensation depends on the wall-loss functional form. For reference, the initial mass concentration of particles in the chamber was 280 μ g m− 3.
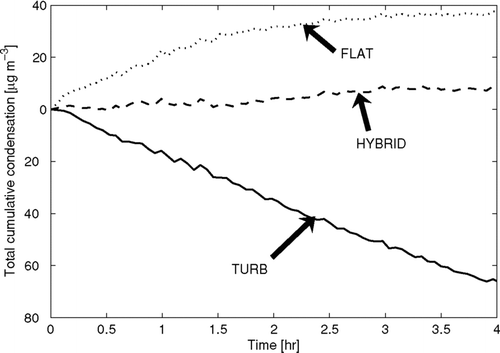
FIG. 5 Average wall loss rates as a function of diameter during the ammonium sulfate experiment for the three wall-loss functional forms and the time-averaged wall-loss rate calculated assuming that there was no condensation/evaporation during the experiment corrected for coagulation (No condensation). The uncertainty in the wall-loss rate due to uncertainty in the coagulation kernel is shown by the lines above and below the no condensation line. In these lines, the entire coagulation kernel was scaled up and down by 20%.
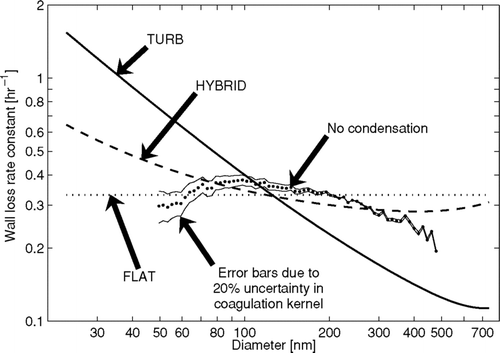
TABLE 1 Limonene oxidation experiments
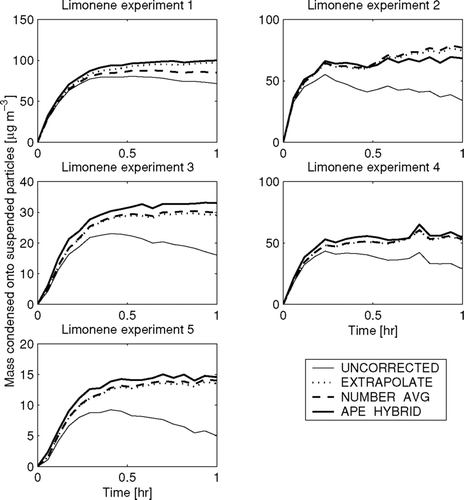
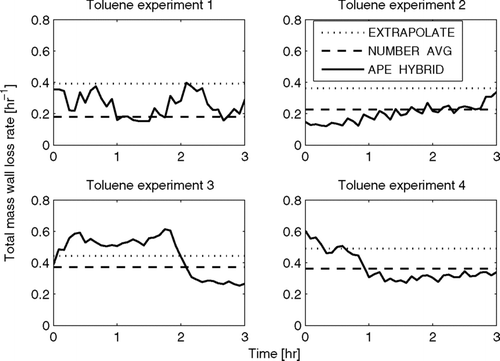
FIG. 6 Total predicted mass wall-loss rate as a function of time in the limonene experiments using the EXTRAPOLATE, NUMBER-AVG, and APE-HYBRID methods. Note that the y-axes change for each experiment.
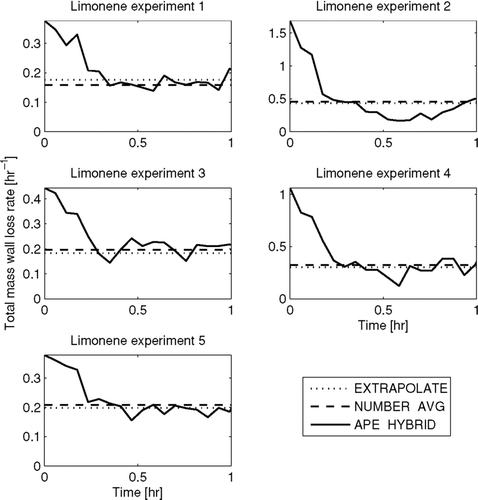
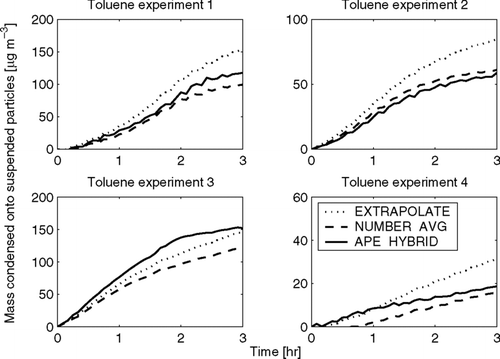
FIG. 7 Total predicted condensation onto suspended particles as a function of time in the limonene-SOA experiments using the EXTRAPOLATE, NUMBER-AVG, and APE-HYBRID methods. Also shown is the total predicted condensation onto suspended particles if wall loss is not corrected for (UNCORRECTED). Note that the y-axes change for each experiment.