Figures & data
FIG. 1 Cross-section view of the vacuum system of the AMS featuring (a) the particle inlet containing the aerodynamic lens system, (b) the ionizing platinum surface and a focusing electrode, (c) oa-TOFMS unit.
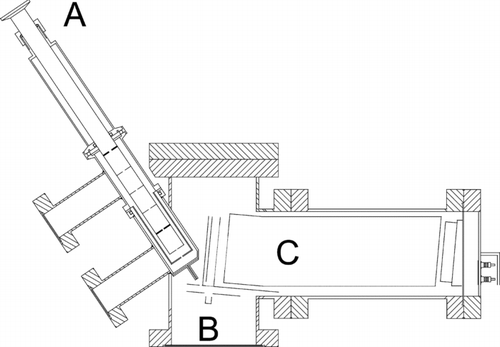
FIG. 2 SIMION (SIMION 2000) calculations of ion trajectories in the instrument: (a) overview of the electrode setup, (b) focusing of ions emitted from the hot Pt surface into the MS, and (c) acceleration and subsequent field-free flight in the TOF section of the MS. The plotted trajectories are for K+ ions. Every 0.5 μs flight-time is marked on the trajectories in (a). The initial kinetic energies were 10 eV in (a) and (c), and 0.1 eV in (b). The following parts are indicated in panel (a): A = platinum surface, B = deflector electrode, and C = time-of-flight tube. Grids with 90% transmission are indicated by thin solid lines.
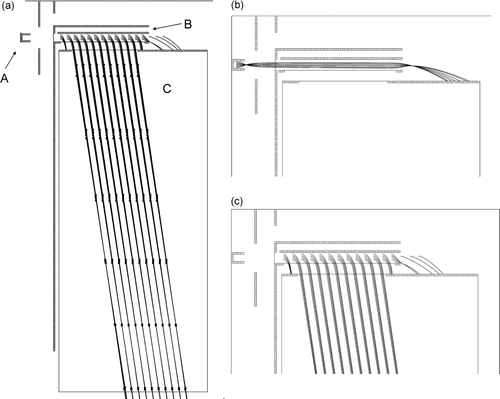
FIG. 3 Example of recorded mass spectra for single laboratory generated aerosol particles with diameters of 70 and 300 nm. Particles were generated from a solution with equal concentrations of sodium, potassium, rubidium, and cesium chloride salts, and particles with a given size were selected with a DMA before entering the AMS.
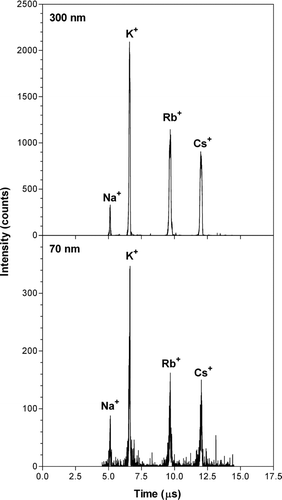
FIG. 4 Distributions of the number of counts observed for individual NaCl particles. The pre-selected diameters used in the experiments are indicated in the panels. The y-axis units are on the same scale.
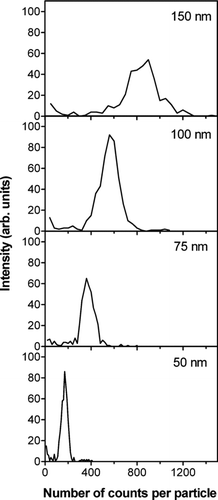
FIG. 5 Average number of ion counts per particle as a function of particle diameter. The particles were generated from solutions of sodium, potassium, rubidium, and cesium chloride salts. The statistical errors were smaller than the size of the symbols used.
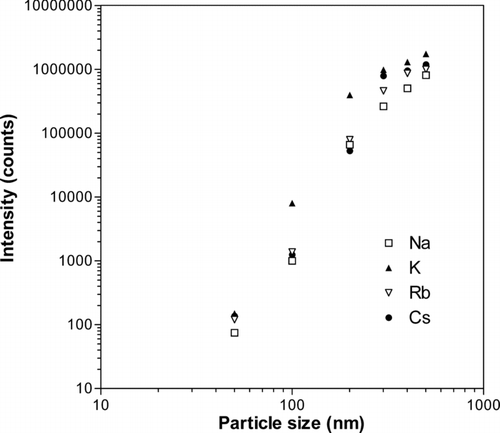
FIG. 6 Examples of mass spectra recorded for particles from ambient air with the AMS. Particles with a diameter of 100 nm were selected with a DMA prior to the AMS. The measurements were performed at the University of Gothenburg Campus on August 24, 2005.
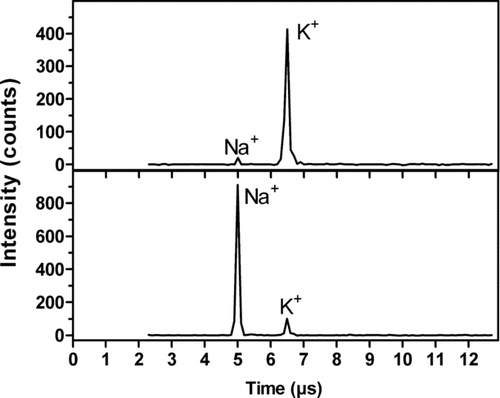
FIG. 7 Na, K, Rb, and Cs concentrations as a function of time during 40 seconds of operation in ambient air for all particle sizes. The measurements were performed at the University of Gothenburg Campus on August 25, 2005.
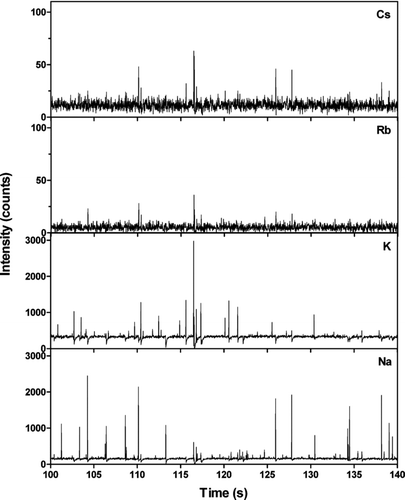
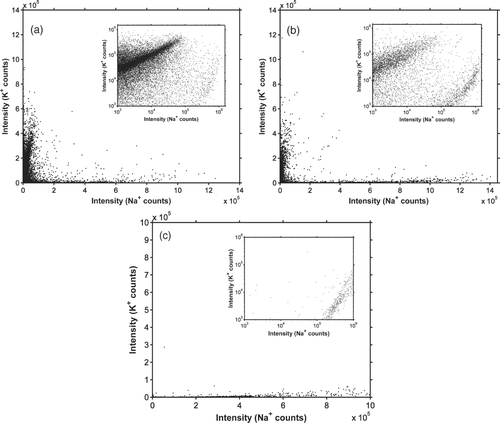
FIG. 8 The number of Na+ and K+ ion counts in individual particles. (a) Sampled from ambient air in Gothenburg from 04:00 to 08:00 on February 20, 2007. (b) Sampled from ambient air in Gothenburg from 15:52 to 19:52 on April 11, 2007. (c) Sampled from a laboratory setup used to mimic the formation of sea spray particles. All particle sizes where let into the AMS during the experiments. The insets show the same data on a logarithmic scale.