Figures & data
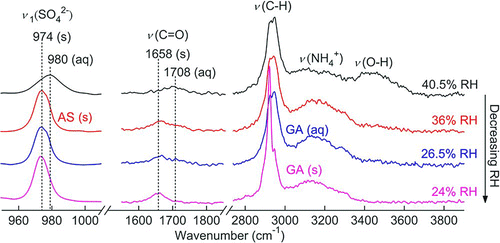
FIG. 1 Hygroscopicity (WSR, mass of water/mass of solute) of (a) AS and (b) AN particles as a function of RH obtained by micro-Raman spectroscopy. Predictions from the E-AIM model are also included. (Figure provided in color online.)
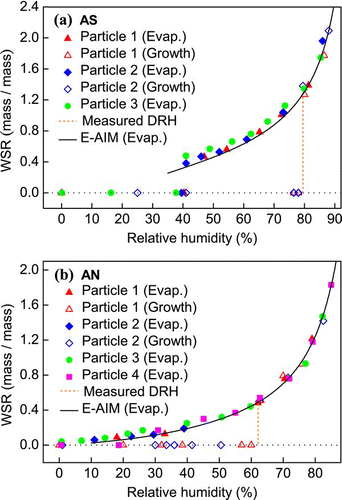
FIG. 2 Hygroscopicity (WSR, mass of water/mass of solute) of (a) MA, (b) GA, and (c) GlyA particles as a function of RH obtained by micro-Raman spectroscopy. Predictions from the UNIFAC model are also included. (Figure provided in color online.)
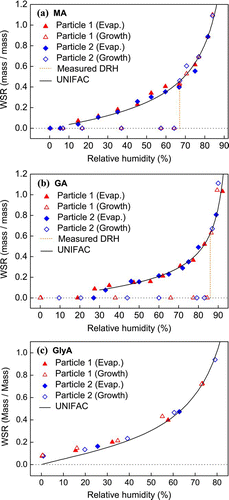
FIG. 3 Hygroscopicity (WSR, mass of water/mass of solute) of AS-MA mixed particles (in 1:1 mole ratio) undergoing (a) evaporation and (b) growth processes obtained by micro-Raman spectroscopy. Data from CitationChoi and Chan (2002) and CitationLing and Chan (2008) and predictions from the ZSR and E-AIM models are also included. (Figure provided in color online.)
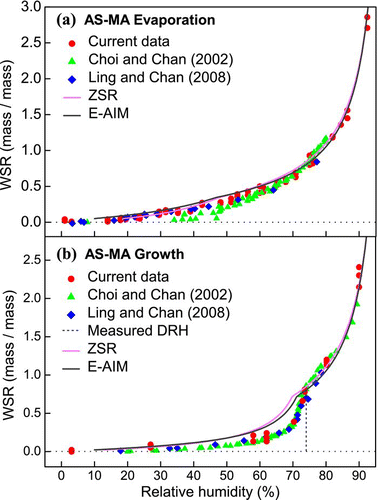
FIG. 4 Hygroscopicity (WSR, mass of water/mass of solute) of AS-GA mixed particles (in 1:1 mole ratio) undergoing (a) evaporation and (b) growth processes obtained by micro-Raman spectroscopy. Data from CitationChoi and Chan (2002) and CitationLing and Chan (2008) and predictions from the ZSR and E-AIM models are also included. (Figure provided in color online.)
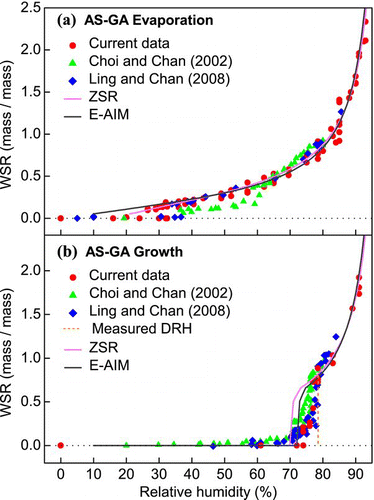
FIG. 5 Raman spectra of a single (a) AS and (b) AN particles upon evaporation. (Figure provided in color online.)
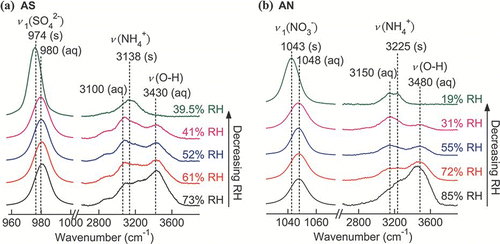
FIG. 6 Changes in normalized fwhh and Raman shift of the (a) sulfate peak (980 cm− 1) of a single AS particle and (b) nitrate peak (1048 cm− 1) of a single AN particle through a humidity cycle, and (c) changes in the Raman shift of the nitrate peak (1048 cm− 1) of three individual AN particles upon crystallization. (Figure provided in color online.)
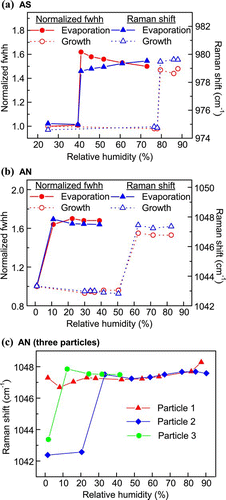
FIG. 7 Raman spectra of a single (a) MA and (b) GA particles upon evaporation. (Figure provided in color online.)
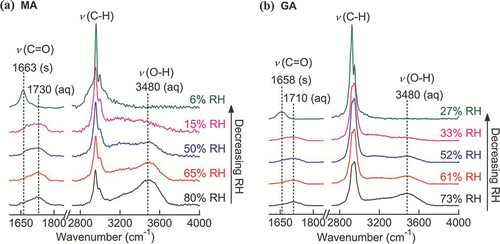
FIG. 8 Changes in normalized fwhh and Raman shift of the C = O peak of single (a) MA and (b) GA particles through a humidity cycle, and (c) a GlyA particle upon evaporation. (Figure provided in color online.)
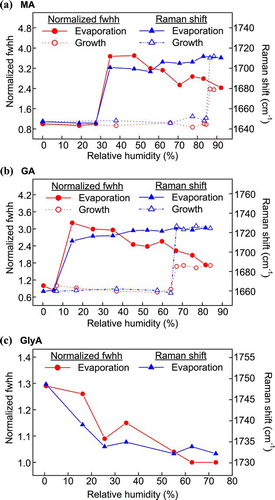
FIG. 9 Raman spectra of a single GlyA particle (a) upon evaporation and (b) under dry and hot conditions. (Figure provided in color online.)
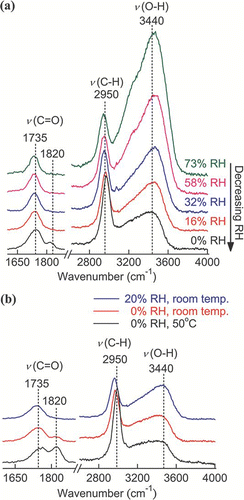
FIG. 10 Changes in (a) the normalized fwhh of the sulfate peak (980 cm− 1) and (b) the intensity ratio of the C = O double-peak (I1730/I1665) of AS-MA mixed particles through a humidity cycle. (Figure provided in color online.)
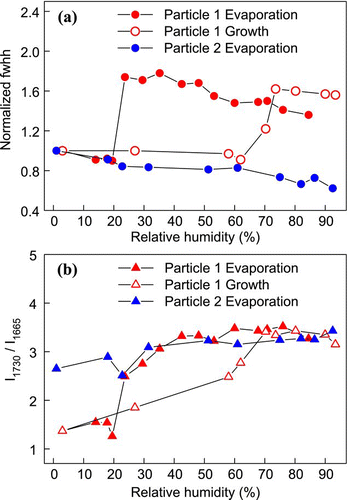
FIG. 11 Raman spectra of a single AS-MA mixed particle upon evaporation. (Figure provided in color online.)
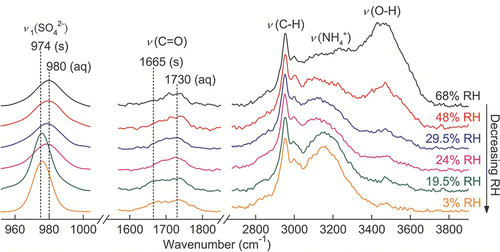
FIG. 12 Changes in (a) the normalized fwhh of the sulfate peak (980 cm− 1) and (b) the intensity ratio of the C = O double-peak (I1708/I1658) of AS-GA through a humidity cycle. (Figure provided in color online.)
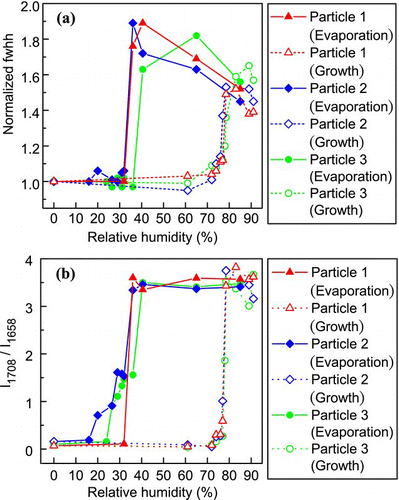
FIG. 13 Raman spectra of a single AS-GA mixed particle upon evaporation. (Figure provided in color online.)