Figures & data
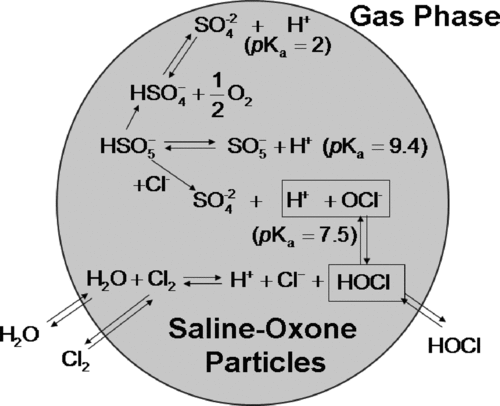
FIG. 2 UV-Visible spectroscopy to monitor the oxidation capability of active chlorine and aerosol acidity in both internally and externally mixed aerosols. For externally mixed aerosol, Oxone particles were collected on the dyed filter containing the preexisting saline or saline-buffer particles. For the internally mixed aerosol, the saline-Oxone aerosol is sampled on the dyed filter using the different sampling systems (F1-F2-F3 and F1-D-F2).
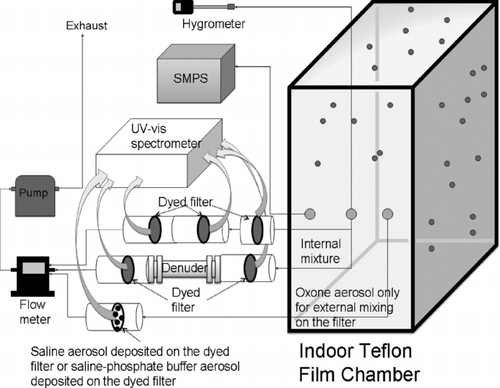
TABLE 1 Experimental conditions to study aerosol reactivity and acidity using UV-Vis spectroscopy for the aerosol sample collected on the dyed filter with metanil yellow or thymol blue a
FIG. 3 FTIR experimental setup to measure the water amount in oxidant particles. The small flow chamber equipped with silicon and ZnSe FTIR windows was placed in the FTIR optical beam path in the transmission mode.
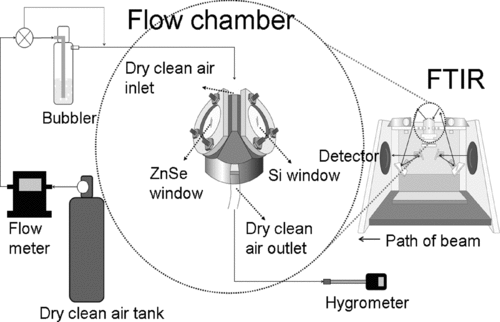
TABLE 2 Experimental conditions to monitor the water content in saline, Oxone, and saline-Oxone particles using FTIR and the estimated water amount in various particles in the two different RH levels at 20% and 70%
FIG. 4 The ΔA’420,oxi (Equation (2)) obtained using different sampling systems (F1-F2-F3 and F1-D-F2) for the internally mixed saline-Oxone aerosol after the Oxone was mixed with saline in the aqueous solution. Error bars in F1 filters are estimated from uncertainties in the SMPS data (6%), and UV-Visible absorbance (4%) and sampling flow rate (1%). Error bars in F2 and F3 filters which evaluate oxidation effects of gas-phase oxidants are estimated from uncertainties in the sampling flow rate and UV-Visible absorbance.
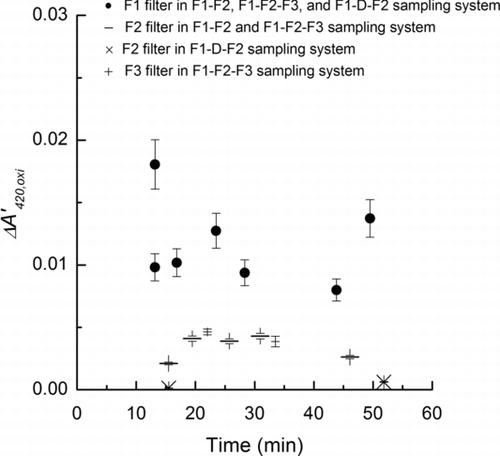
FIG. 5 Time profile of ΔA’420,oxi (Equation (2)) of the external mixture of Oxone, saline-Oxone, saline-Oxone with phosphate buffer aerosol. Error bars were estimated from uncertainties in the sampling device, balance, SMPS data, and UV-Visible absorbance.
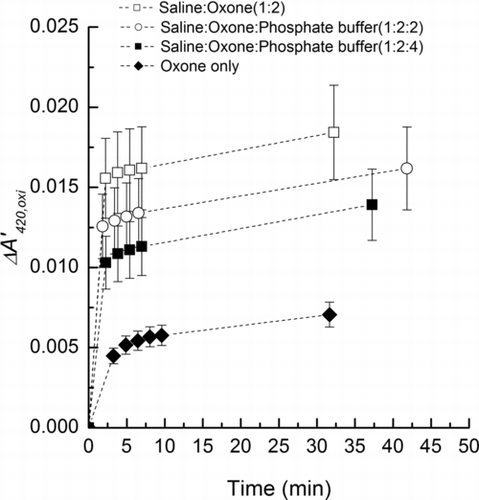
FIG. 6 FTIR spectra of the tested buffers (sodium bicarbonate and phosphate buffer) and the subtracted FTIR spectra ([spectrum of Saline/Oxone/Buffer] − [spectrum of Saline/Oxone])
![FIG. 6 FTIR spectra of the tested buffers (sodium bicarbonate and phosphate buffer) and the subtracted FTIR spectra ([spectrum of Saline/Oxone/Buffer] − [spectrum of Saline/Oxone])](/cms/asset/1ade0ccc-45bd-4c82-8725-af9ea7de95b2/uast_a_507612_o_f0006g.gif)
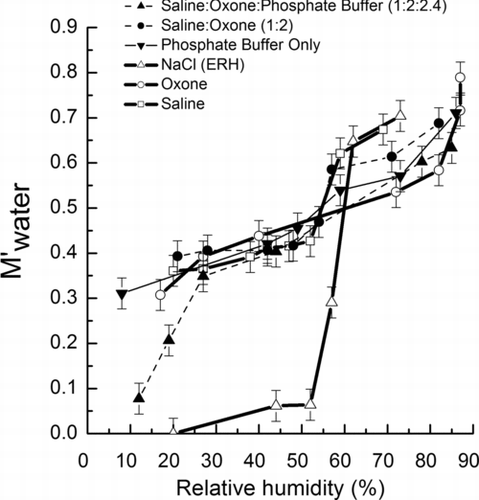
FIG. 7 The M′ water (Equations (Equation5) and (6), μg/μg) of NaCl, saline, Oxone, and saline-Oxone aerosols as a function of decreasing humidity. Error bars were estimated from uncertainties in the FTIR absorbance at O-H band and balance.