Figures & data
Figure 1 Schematic of the system used to examine back charging reactions between TMA vapor and charged amino acid clusters. Amino acid clusters were produced by electrospray ionization, and TMA vapor was introduced into the gas phase at varying concentration by nebulizing TMA solution.
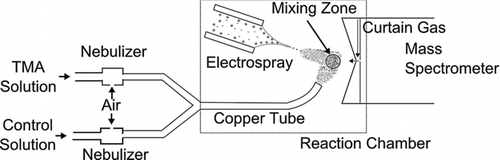
Figure 2 Selected mass spectra obtained when producing charged clusters of Asparagine with 3 different TMA vapor concentrations. The TMA concentration present during data collection is proportional to the flow rate of TMA solution, which is noted in the upper right corner of each plot. (Figure provided in color online.)
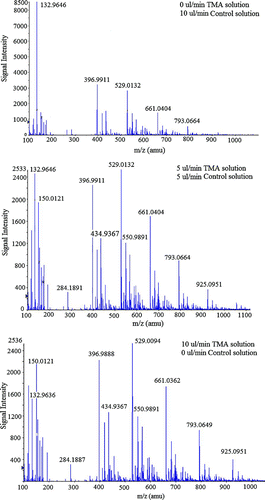
Figure 3 Plots used in determination of the relative back reaction rate for protonated amino acid monomer, dimer, and trimer ions with TMA vapor molecules. The labels on the abscissa and ordinate are described in the text.
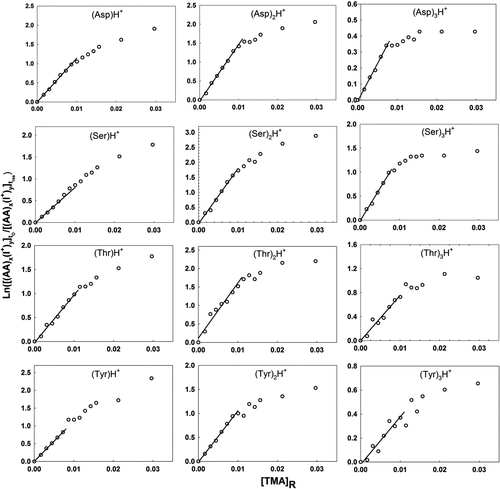
Figure 4 Plots used in determination of the relative back reaction rate for sodiated, potassiated, and trimethylammoniated amino acid monomer, dimer, and trimer ions with TMA vapor molecules. Negative slopes for the trimethylammoniated clusters indicate that TMA vapor molecules can stick to cluster ions upon collisions. The labels on the abscissa and ordinate are described in the text.
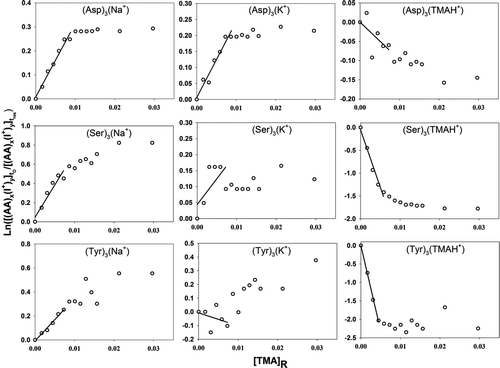
Figure 5 Plots used in determination of the relative back reaction rate for doubly protonated amino acid cluster ions with TMA vapor molecules. The labels on the abscissa and ordinate are described in the text.
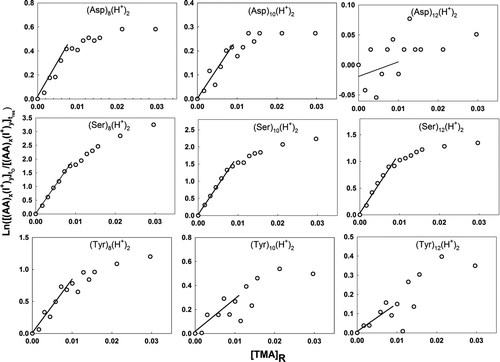
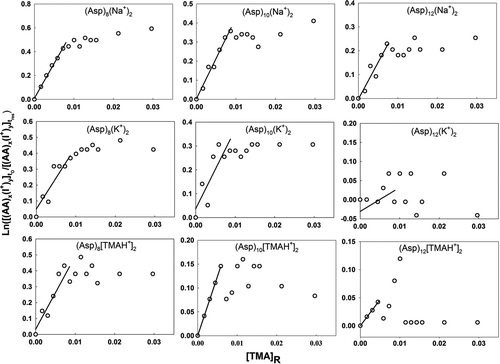
Figure 6 Plots used in determination of the relative back reaction rate for doubly sodiated, potassiated, and trimethylammoniated asparagine cluster ions with TMA vapor molecules. The labels on the abscissa and ordinate are described in the text.