Figures & data
Figure 2 A single linear relationship between particle concentration in air (#/cm3) and water (#/ml), i.e., one universal line, for various types of insoluble particles and their mixture.
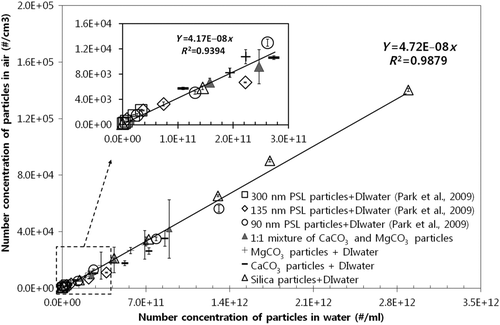
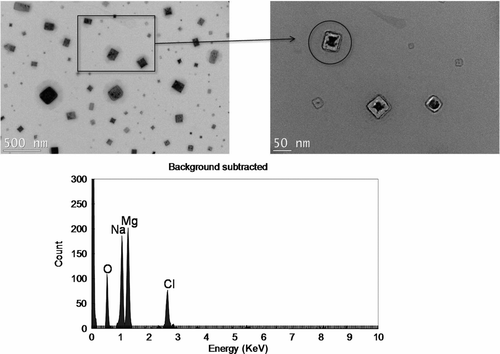
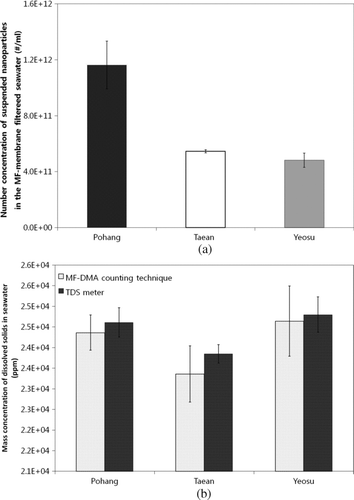
Table 1 The geometric mean diameters (GMDs) of airborne particles formed from dissolved solids (NaCl, KCl, MgCl2, and CaCl2) in DI water
Figure 3 Size distributions of particles aerosolized from the CaCl2 water solution as a function of solution concentration (ppm).
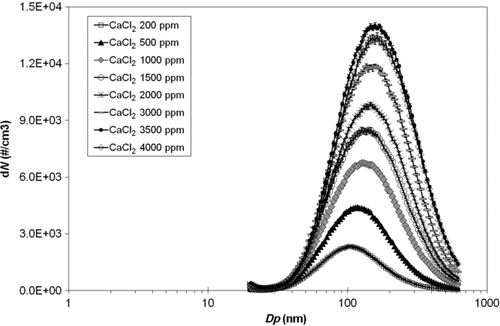
Figure 4 (a) Size distribution as a function of solution concentration; and (b) the relationship between the particle mass concentration in air (ug/m3) and water (ppm) for artificial seawater at various concentration levels.
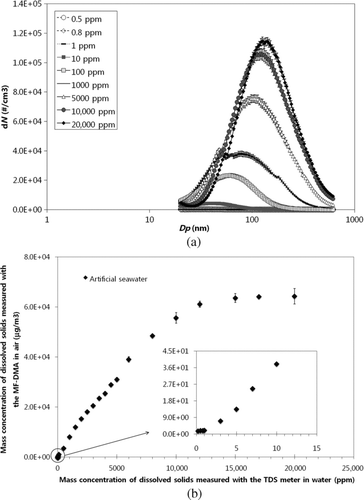
Figure 5 A relationship between particle mass concentration in air (ug/m3) and water (ppm) for various dissolved solids (artificial seawater, NaCl, KCl, CaCl2, and MgCl2) and their mixtures (a 1:1 mixture of NaCl and MgCl2, a 1:1 mixture of NaCl and KCl, and a 1:4 mixture of NaCl and MgCl2).
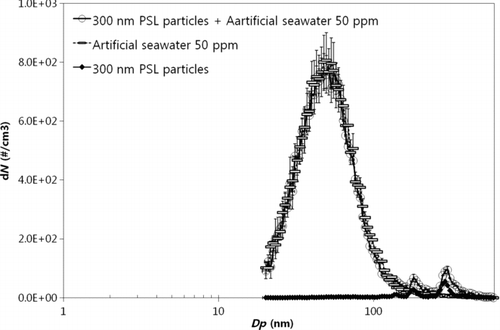
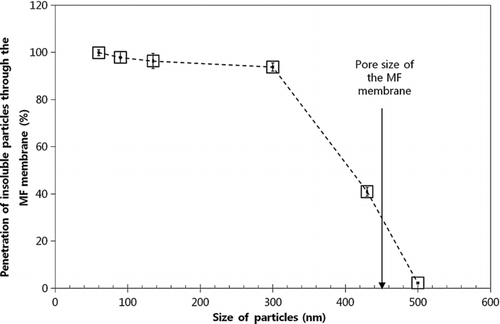
Figure 7 Penetration of insoluble particles (60 nm, 90 nm, 135 nm, 300 nm, 430 nm, and 500 nm PSL particles) through the MF membrane with 0.45 μm pores.