Figures & data
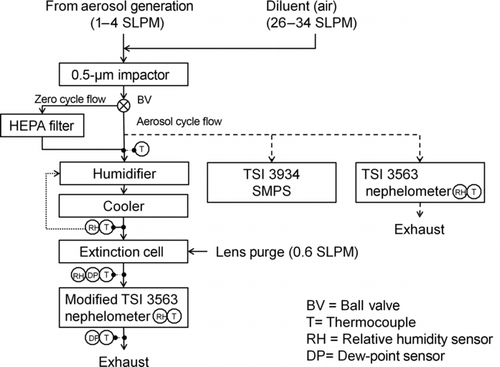
TABLE 1 Aerosol types, aerosol generation method, and purpose for tests
FIG. 2 Short Path Extinction Cell (SPEC) overview: M is a silver plated mirror, L1 and L2 are achromatic lenses, BS is a beam splitter, PD are photo detectors, HD1 and HD2 are holographic diffusers, and LS is the light source.
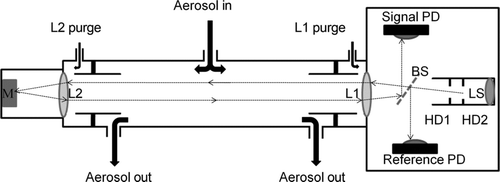
FIG. 3 Evaluation of the optical modified and temperature controlled nephelometer. (a) Measured Rayleigh scattering values (σ sg) for the calibration gases air (zero), CO2 (Span1), and SF6 (Span2) for standard TSI 3563 nephelometer (circles) and modified instrument (squares). Dashed lines indicate theoretical values. (b) Comparison of scattering values from the modified nephelometer and the wavelength interpolated unmodified nephelometer determined with ammonium sulfate aerosol at different aerosol concentrations. The dashed line indicates ideal correlation. For all three wavelengths, the instruments differ less than 1.5%. (Color figure available online.)
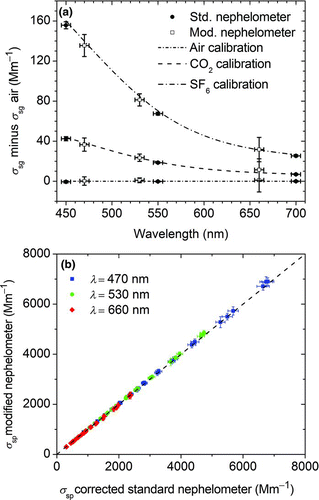
FIG. 4 Root mean square (rms) noise values for particle-free air extinction and scattering as a function of wavelength and sample averaging time for the constructed extinction cell and the modified nephelometer, respectively. (Color figure available online.)
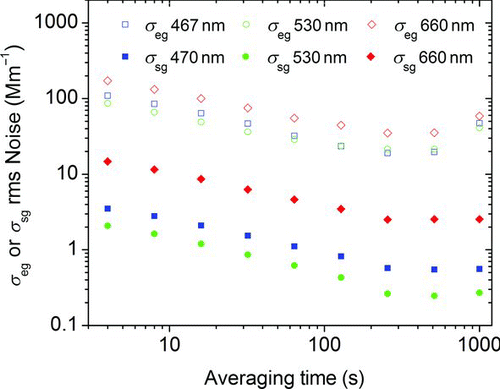
TABLE 2 Linear regression coefficients obtained for a regression between the measured scattering and measured extinction for dry ammonium sulfate to obtain correction factors for nonidealities in the extinction cell. All R 2 values were greater than 0.99 and the regression equation was σ ep, corrected = σ sp = Intercept + Slope × σ ep, measured
TABLE 3 Linear regression coefficients from comparison between the modeled and measured scattering (σ sp, model = Intercept + Slope × σ sp, measured) of dry ammonium sulfate. All R 2 values were greater than 0.99
FIG. 5 RH sensor agreement at RH set point values of (a) 34% RH, (b) 50% RH, (c) 90% RH, and (d) 97.5% RH. V1 and V2 are the capacitive-based RH values. DP1 and DP2 are the dew-point-based RH values calculated with colocated dry-bulb temperature measurements.
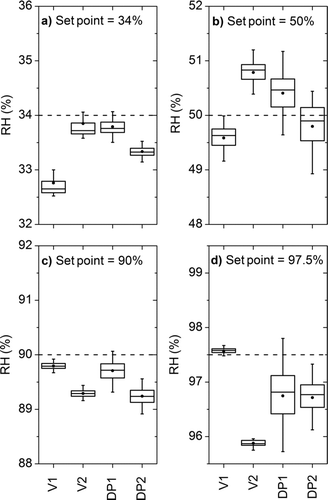
FIG. 6 Measured scattering (σ sp) and extinction (σ ep) coefficients for ammonium sulfate aerosol as a function of RH. The modeled values and their uncertainties are indicated as solid and dashed lines, respectively. (Color figure available online.)
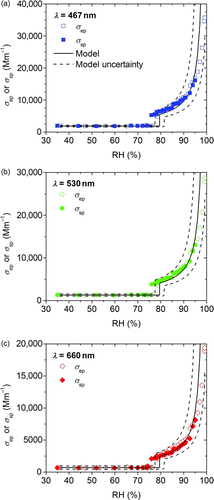
FIG. 7 Measured scattering (σ sp) versus extinction (σ ep) coefficients at three wavelengths for different 327-nm diameter light-absorbing PSL microspheres concentrations. A linear best fit (dashed line) was used to determine the single scattering albedo ω. (Color figure available online.)
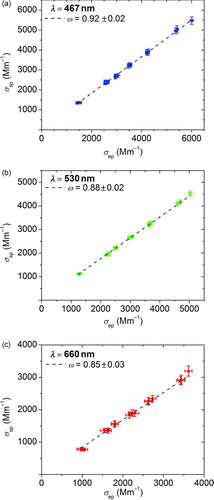
FIG. 8 Optical properties of nigrosin as a function of RH. Extinction (σ ep) and scattering (σ sp) for 467, 530, and 660 nm are shown in (a), (b), and (c), respectively; calculated absorption (σ ap) by difference in σ ep and σ sp is shown in (d). (Color figure available online.)
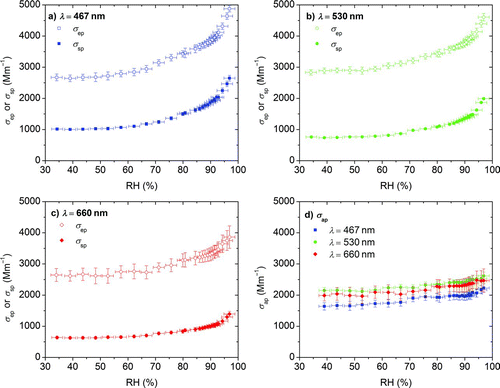
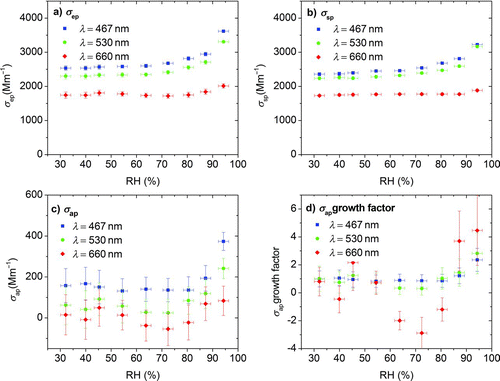
FIG. 9 Optical properties at controlled RH conditions for primary OC aerosol generated by pyrolysis of biomass. Extinction (σ ep), scattering (σ sp), and absorption (σ ap) by difference for three wavelengths are shown in (a), (b), and (c); the hygroscopic absorption growth factor is shown in (d). (Color figure available online.)