Figures & data
FIG. 1 Kinetics of the uptake of 40-ppm TEA by particles of ammonium oxalate, ammonium bisulfate, ammonium sulfate, ammonium chloride, ammonium nitrate, sodium chloride, and sodium sulfate. (Color figure available online.)
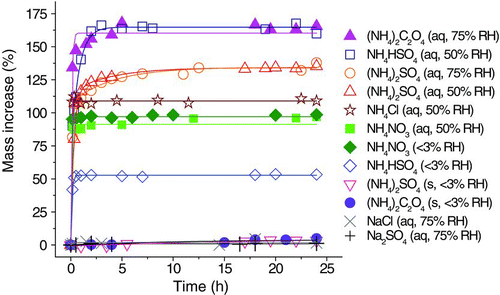
FIG. 2 (a) Temporal changes in Raman signals of an aqueous ammonium chloride droplet at 40-ppm TEA and (b) comparison of the Raman spectra of a solid particle of ammonium chloride, the dried particle of the reacted ammonium chloride droplet in TEA-free EDB chamber, and a solid particle of TEAHCl. (Color figure available online.)
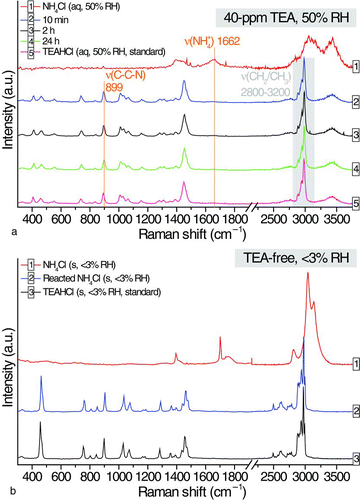
TABLE 1 Summary of the reaction conditions, the particle mass changes on a dry basis and the theoretical mass ratios
FIG. 3 Kinetics of the uptake of 40-ppm TEA by TEAHBS droplets at 50 and <3% RH. (Color figure available online.)
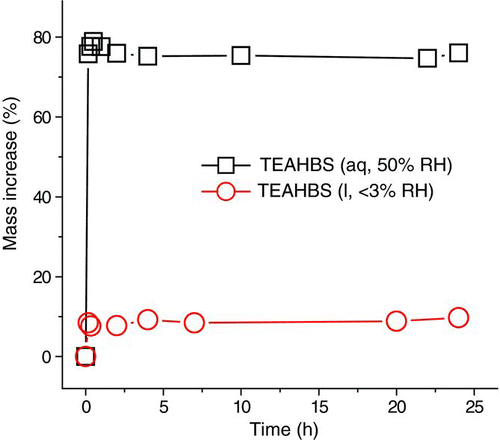
FIG. 4 Temporal changes in (a) Raman signals of the aqueous TEAHBS droplet at 40-ppm TEA (50% RH); (b) Raman signals of the aqueous TEAH sulfate droplet during decomposition in the TEA-free EDB chamber (50% RH); (c) Raman signals of the partially decomposed TEAH sulfate particle during decomposition at <3% RH and its Raman spectrum after re-humidification. (Color figure available online.)
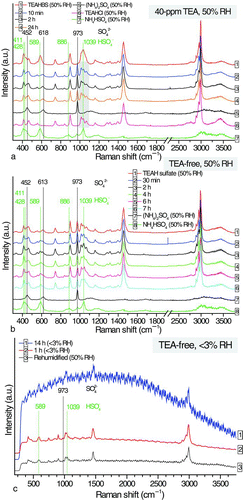
FIG. 5 (a) Temporal changes in the Raman signals of an amorphous ammonium bisulfate solid particle at 40-ppm TEA (<3% RH) and (b) comparison of the Raman spectra of the dried particle of the reacted ammonium bisulfate in TEA-free EDB chamber, a 50 mol% ammonium sulfate/TEAHBS mixed particle, a crystalline ammonium sulfate solid particle, and a TEAHBS droplet, all at <3% RH. (Color figure available online.)
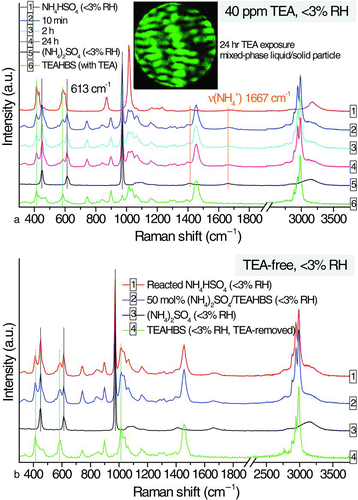
FIG. 6 Uptake kinetics of aqueous ammonium sulfate particles at 3-ppm and 700-ppb TEA, all at 50% RH. (Color figure available online.)
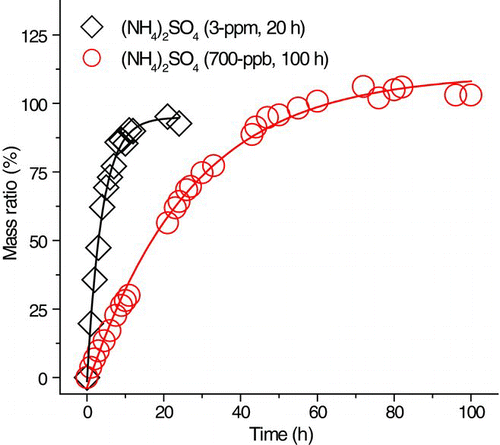
FIG. 7 Normalized sulfate peak area ratio against mol% ammonium sulfate. (Color figure available online.)
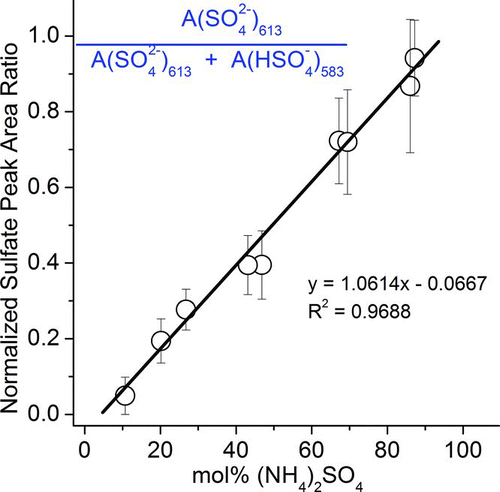
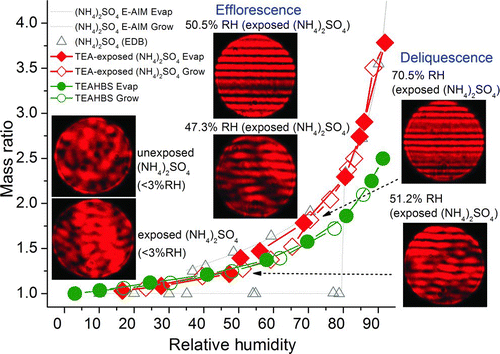
FIG. 8 Hygroscopicities of E-AIM predictions of ammonium sulfate and EDB-measurements of ammonium sulfate particles, the 3-ppm TEA-exposed (20 h, 50% RH) ammonium sulfate particle and a TEAHBS particle, and Mie scattering patterns of the 3-ppm TEA-exposed ammonium sulfate particle. (Color figure available online.)