Figures & data
TABLE 1 Signal contribution (%) and possible ion formula in unit-mass-resolution (UMR) mass spectra of organic particles from the burning of IS, IC, and MC
FIG. 1 Average unit-mass-resolution (UMR) mass spectra of organic compounds in particles from burning three types of incense: incense stick (IS), incense coil (IC), and mosquito coil (MC). Three groups of ions, including homologous series (Group I), sugar- and aromatics-related ions (Group II), and lignin-related ions (Group III), are chosen for further analysis.
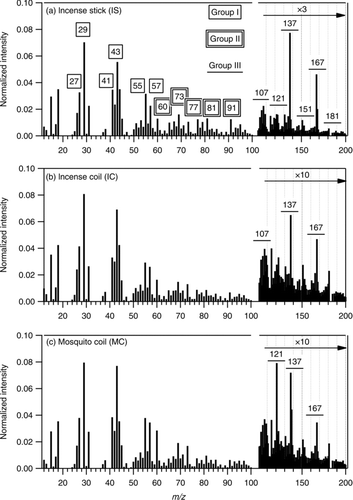
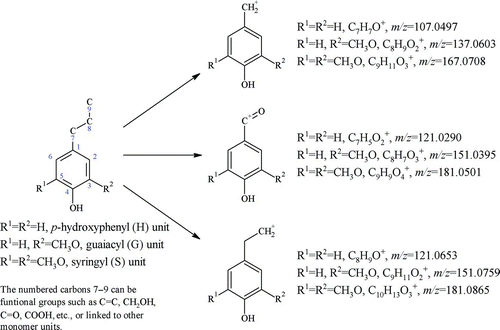
FIG. 2 High-resolution (HR) mass spectra of lignin-related ions (Group III) from burning three types of incense. A peak in the region of m/z 107 (before C7H7O+), more obviously shown in panels (b) IC and (c) MC, should be C6H3O2 +. It is not included in the fitting here since its peak is much smaller than the others (C7H7O+ and C8H+ 11). At m/z 167, the ion C8H7O4 + can be due to phthalates (McLafferty and Turecek Citation1993) but the other ion (C9H11O3 +) is due to organics from incense burning and it dominates. (Color figure available online.)
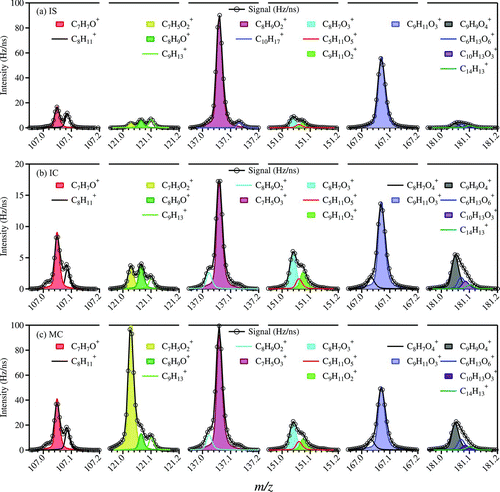
FIG. 3 Example of correlations: the mass spectrum from mosquito coil (MC) with mass spectra from (a) levoglucosan, (b) lignin powder, and (c) PMF-resolved BBOA. Pearson's r values and slopes in the correlations of mass spectra from different BBOA, including particles from incense burning, with those of (d) levoglucosan, (e) lignin powder, and (f) PMF-resolved BBOA.
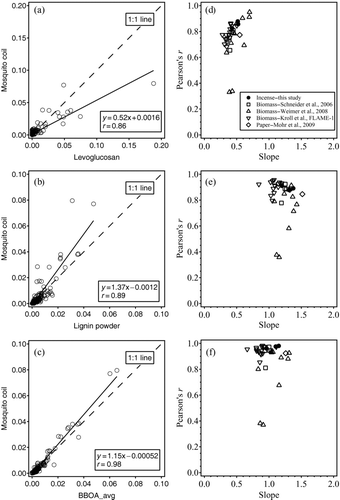
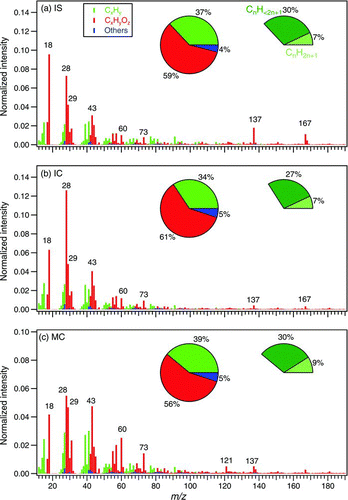
FIG. 5 High-resolution mass spectra of particulate organics from burning (a) incense stick (IS), (b) incense coil (IC), and (c) mosquito coil (MC), using the modified fragmentation table (frag_18/28_v_i). The inserted pie charts on the left represent the percentages of hydrocarbon ions (CxHy), oxygenated ions (CxHyOz), and others (N- and S-containing compounds). See Section 3.3 for description of these three categories. The pie charts on the right represent the percentages of ions with molecular formula of C n H2n+1 (saturated) and C n H<2n+1 (unsaturated or cyclic). (Color figure available online.)