Figures & data
TABLE 1 Aerosol Composition Characteristics of Cigarette Smoke: The aerosol phase is composed of semivolatile organics, and EC makes up <1% of the total organic carbon (TOC). O/C ratio of mainstream ETS is larger than sidestream smoke
FIG. 1 Particle Size distributions from mainstream and sidestream ETS. 1R5F cigarettes form particles of larger modes in both mainstream and sidestream ETS. Because the activation diameters for both cigarette types produce aerosol of similar hygroscopicity (κ ∼ 0.15), ULN 1R5F cigarettes will produce more aerosol that activate and form droplets.
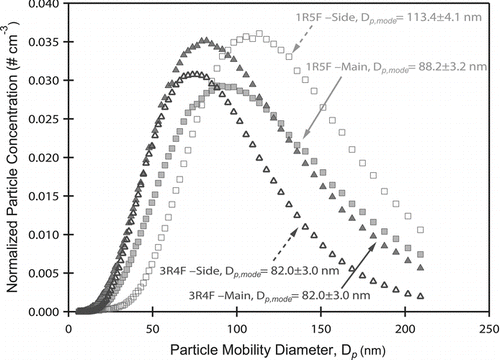
FIG. 2 Exemplary volatility data from Catalytic Stripper (CS) measurements. Log normal particle distribution of ETS and nonvolatile aerosol are measured. The semivolatile distribution is inferred. Inset graph shows the semivolatile fraction as a function of particle mobility diameter. The majority of 3R4F aerosol is semi-volatile and evaporates at temperatures below 300°C. (Color figure available online.)
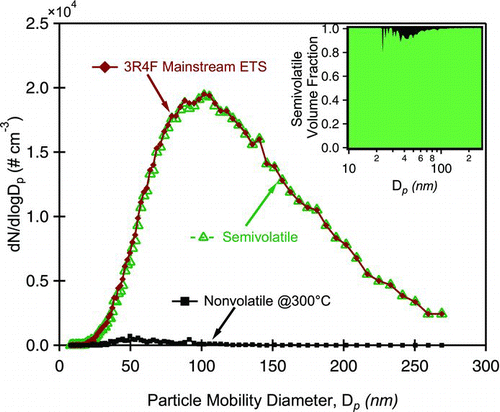
FIG. 3 Exemplary m/z 43 normalized UMR mass spectra of LN 3R4F (darker color) to ULN 1R5F (lighter color) mainstream ETS. Prominent peaks where the ratio LN to ULN mass spectra, R, is (a) <0.95 and (b) >1.10 are shown. (Color figure available online.)
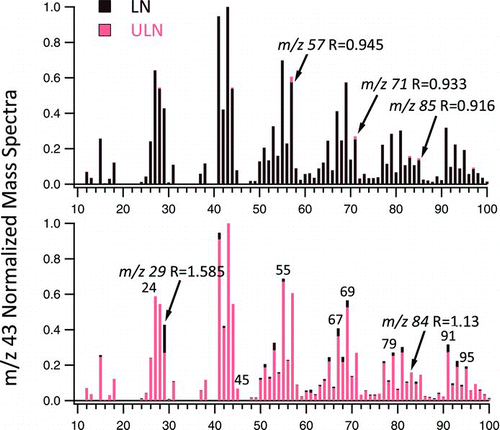
FIG. 4 Exemplary m/z 43 normalized UMR mass spectra of ULN 3R4F main (darker color) to ULN 3R4F (lighter color) sidestream ETS. The m/z 43 is the most abundant fragment in both cigarette types but is formed less in Main (8.5%) versus Side (9.8%) stream smoke. (Color figure available online.)
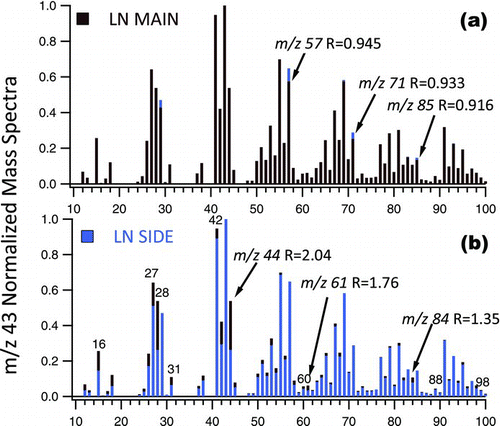
FIG. 5 Activated fraction (CCN/CN) versus particle mobility diameter of mainstream ETS for s c at 0.85% (closed) and 0.54% (open). (NH4)2SO4 calibration data (circles) is highly soluble in water and shown for comparison. LN 3R4F (triangles) have slightly smaller activation diameters, d p50, compared to ULN IR5F (squares) aerosol. κ (∼0.15) of both cigarette types are consistent with partially soluble organic aerosol components.
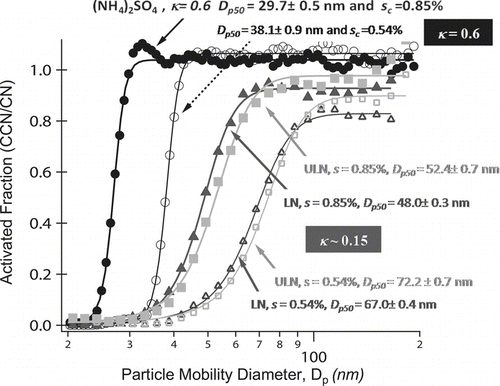
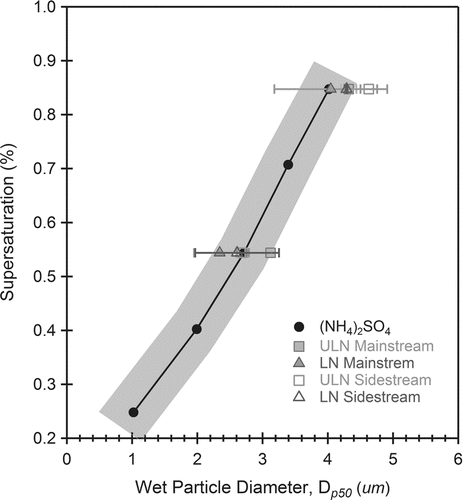
FIG. 6 Droplet growth of LN and ULN ETS. Both LN 3R4F (triangles) and ULN IR5F (squares) aerosol grow to similar sizes as (NH4)2SO4 (circles) at activation in the CCNC instrument. This indicated similar growth kinetics. Despite being less hygroscopic (κ < 0.6, Table 2), organic ETS have the same droplet growth rates as soluble (NH4)2SO4 particles. The shaded area is the region of measurement uncertainty ±0.5 μm.