Figures & data
FIG. 1 Design of the thermal denuder (TD). SOA from the flow tube reactor (FTR) can be directed to either port (A) for SMPS analysis prior to denuding or port (B) for SMPS analysis after denuding. Before the SOA reaches port (B) it either passes through the denuder or a bypass line. (Color figure available online.)
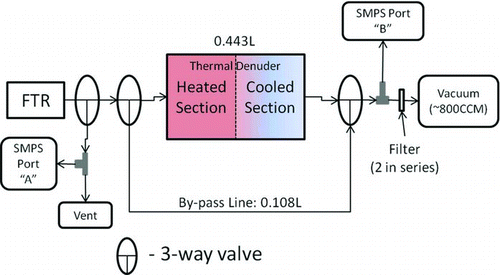
FIG. 2 Percentage of TSP remaining after the SOA passes through the TD, relative to TSP in the bypass line. Bypass TSP is noted above each column, and this value corresponds to 100% for the respective experiment. The TSP remaining is divided into monomer (light) and oligomer (dark) mass contributions. The black error bars represent the 95% confidence interval for total TSP measurement with the SMPS (relative to the bypass TSP). White error bars show the high and low values calculated for the monomer versus oligomer content.
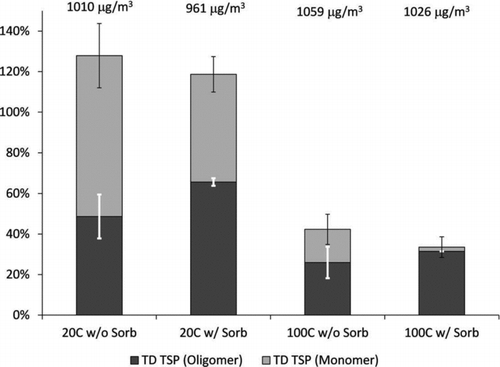
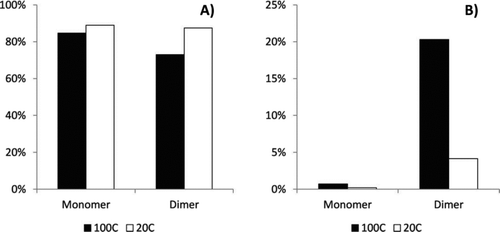
FIG. 3 Calculated unvolatilzed fraction (UF) of representative monomer and dimers, (a) monomers and (b) dimers, after heating from 20°C to 100°C. Also shown are experimental measurements based on the “with adsorbent” data in .
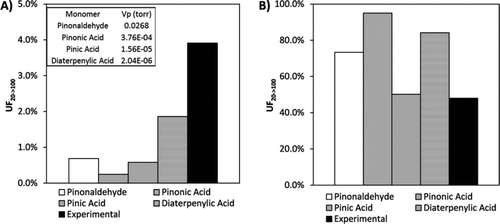
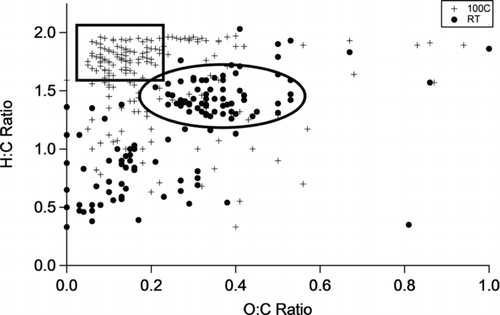
FIG. 4 van Krevelen plots comparing mass spectra for the 20°C and 100°C samples obtained without adsorbent. Assigned formulas represented by circles were observed in the 20°C sample but not the 100°C sample. Assigned formulas represented by crosses were observed in the 100°C sample but not the 20°C sample.