Figures & data
FIG. 1 Experimental setup for aqueous photooxidation and droplet evaporation. Reaction samples were also analyzed by ion chromatography (IC), electrospray ionization mass spectrometry (ESI-MS), IC ESI-MS, and for total organic carbon (TOC). (Color figure available online.)
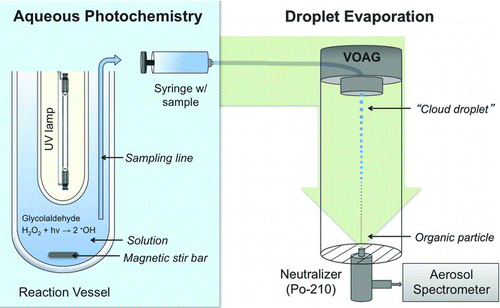
TABLE 1 Particle geometric diameter (D p), concentration-weighted densities (ρ), SOAaq mass yields, and their corresponding uncertainty (Δ) as a function of reaction time (n ≥ 3, 1 mM glycolaldehyde and ∼10−12 M OH radicals)
FIG. 2 Product concentrations from 1 mM glycolaldehyde and OH radicals (∼10−12 M), by ion chromatography (n = 3) for this work and for concentrations obtained by Perri et al. (Citation2009). Note that succinic plus malic acid as well as malonic plus tartaric acid co-elude and were quantified as succinic and malonic acid, respectively (Tan et al. Citation2009). Glyoxylic acid converts to formic acid in samples awaiting analysis (not shown). (Color figure available online.)
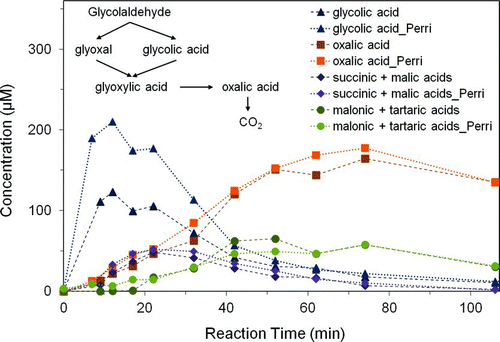
FIG. 3 (a) IC-ESI-MS spectra of a mixed standard: (A) glycolic acid (m/z − 75), (B) formic acid (not detectable by ESI-MS) and residual glycolic acid from peak A, (C) succinic acid (m/z − 117), (D) malonic acid (m/z − 103), (E) contaminants, including sulfate (m/z − 97), and (F) oxalic acid (m/z − 89). IC-ESI-MS spectra of samples taken from the reaction of 1 mM glycolaldehyde + OH radical at 17 min (b) and 52 min (c) reaction times: (A) glycolic acid (m/z − 75), (B) peak with retention time of formic acid, (C) succinic acid (m/z − 117) and malic acid (m/z − 133), (D) malonic acid (m/z − 103) and tartaric acid (m/z − 149), (E) peak with retention time of sulfate (m/z − 97), (F) oxalic acid (m/z − 89), and (G) high-molecular-weight compounds.
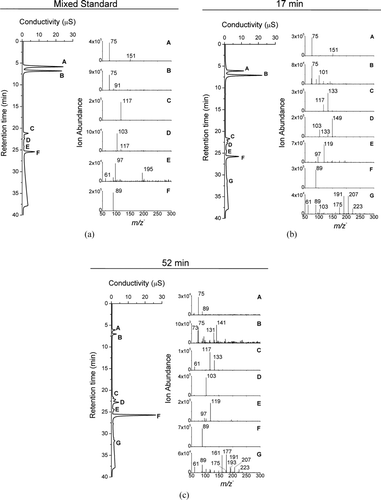
FIG. 4 (a) IC time profile of succinic and malic acid concentration from the reaction of 1 mM glycolaldehyde + OH radicals (∼10−12 M), and overlaid IC-ESI-MS ion abundance time profiles for m/z− 117 (succinic acid) and m/z− 133 (malic acid). (b) IC time profile of malonic and tartaric acid concentration, and overlaid IC-ESI-MS ion abundance time profiles for m/z− 103 (malonic acid) and m/z − 149 (tartaric acid). (Error bars represent the pooled coefficient of variation between experiments.)
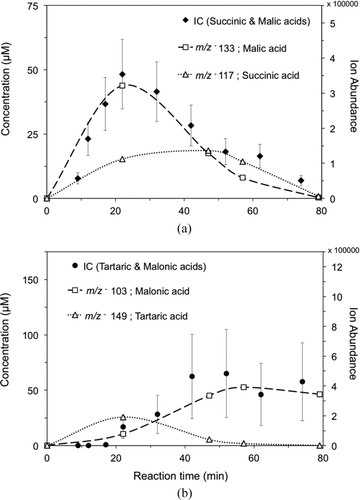
FIG. 5 Mass of residual particles (PM mass) formed from droplet evaporation of organic acid standard solutions of acetic, oxalic, succinic, glutaric, and tartaric acid. OM mass(droplet) is the mass of organic matter in the droplet. Labels include liquid vapor pressures estimated using the SIMPOL group contribution method (Pankow and Asher Citation2008). Slopes and r2 values are reported in (D d = 18.3 ± 0.4 μm; RH = 13 ± 2%; T = 24.1 ± 0.3°C). (Color figure available online.)
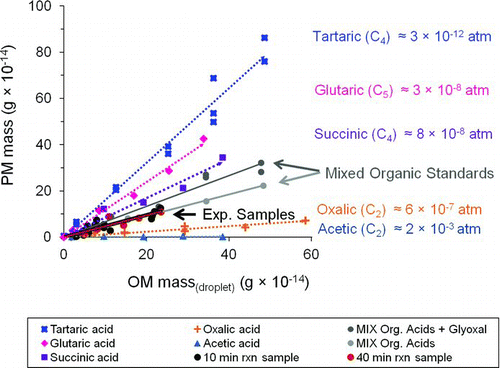
FIG. 6 Ratio of residual particle mass (PM mass) to droplet organic matter (OM mass) versus log(p L°), where p L° is the liquid vapor pressure. (PM mass/OM mass) values are the slopes from (see also ). (1) PM mass/OM mass values from , where densities were calculated from organic species. (2) PM mass/OM mass values computed using densities calculated with an upper-bound estimate of retained water. Gray arrow indicates the PM mass/OM mass of glycolaldehyde SOAaq.
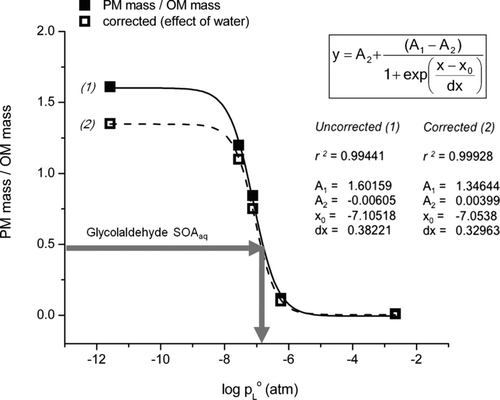
FIG. 7 Volume (D p 3) of residual particles and total organic carbon (TOC) in droplets, from droplet evaporation of oxalic acid and ammonium oxalate standard solutions. Liquid vapor pressure of ammonium oxalate p L° (US EPA: EPI Suite™ 2010) (D d = 18.3 ± 0.4 μm; RH = 13 ± 2%; T = 23.8 ± 0.3°C). The clear difference between oxalic acid and ammonium oxalate (and day-to-day reproducibiliy of oxalic and succinic acid results) provide confidence that the VOAG system was free of ammonium contamination.
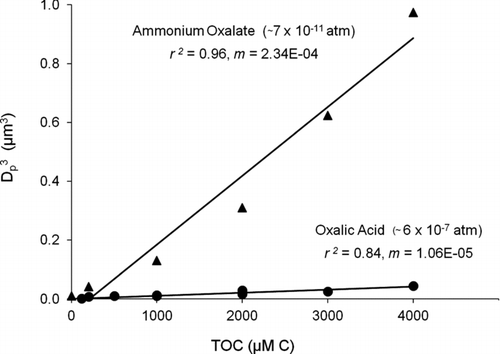
TABLE 2 Slopes (m), coefficients of determination (r2 ), liquid vapor pressures (p L°), and enthalpies of vaporization (ΔH vap)
FIG. 8 Proposed mechanism for the formation of glycolaldehyde oligomers through hemiacetal formation (a) and aldol condensation (b).
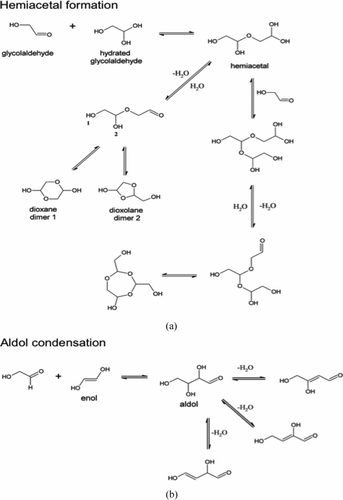
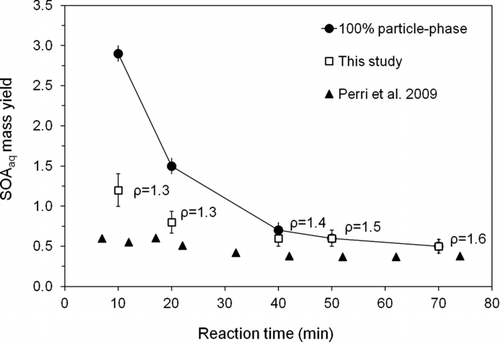
FIG. 9 SOAaq mass yields from the reaction of 1 mM glycolaldehyde + OH radicals (∼10−12 M). Squares are mass yields calculated in this study using concentration-weighted densities and assuming spherical particles (n ≥ 3; D d = 18.3 ± 0.4 μm; RH = 10 ± 1%; T = 23.7 ± 0.7°C). Triangles are yields estimated by Perri et al. (Citation2009) using concentrations of species measured in the reaction vessel and estimating the fraction of each that would remain in the particle phase from atmospheric measurements. Circles are upper-bound yields obtained if all the organic mass in the droplet remained in the residual particle, calculated using IC quantification of organic acids and model predictions of remaining glycolaldehyde and glyoxal and neglecting unquantified products. (Error bars for open squares represent the pooled coefficient of variation for identical time points across experiments. Error bars for circles are from error propagation).