Figures & data
FIG 1 Area constructions derived from the Kelvin curve. Solid curve is the Kelvin curve for water from EquationEquation (1). Horizontal dashed line is for a water vapor saturation ratio of 2 (relative humidity = 200%). The point of intersection marks the critical drop size, g*, consisting of the seed particle plus n*=g*− v seed/v 1 molecules of condensed water. See text for interpretation of labeled areas R 1−R 4 and the tangent line.
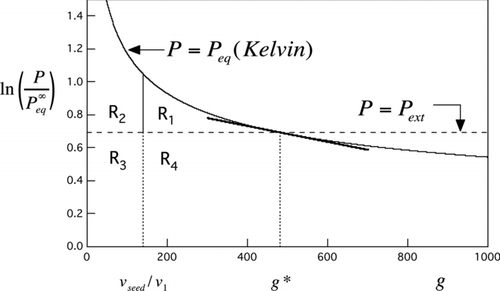
FIG 2 Scaled nucleation barrier profiles from EquationEquation (7) for several seed volume fractions (f=n seed/g*). Curves, top to bottom: homogeneous nucleation case (f=0), an intermediate heterogeneous nucleation case (f=0.25), and the Kelvin limit (f=1).
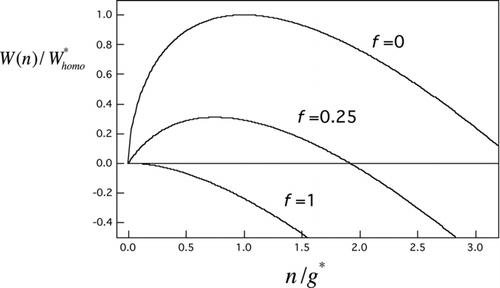
FIG 3 Typical behavior of the mean first passage time (MFPT) to reach a given cluster size as a function of that size. Here, n is the number of molecules condensed onto the seed. Region I, quasi-equilibrium between clusters of precritical size. Region II, inflection point at the critical size. Region III, rapid-growth regime. Calculations are for heterogeneous nucleation of l-menthol on a 1.5-nm-diameter seed. S ext=86.0, W*/kT=18.1, J 1=1 s−1.
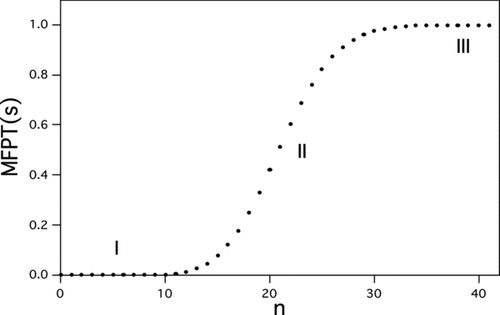
FIG 4 Nucleation rates for menthol. Solid curve is the Kelvin limit. Dashed lines and curves are contours of constant nucleation rate. Horizontal lines: contours of constant homogeneous nucleation rate: top to bottom, J homo= 100, 1, and 0.01 cm−3s−1. Dashed curves give similar contours for the heterogeneous nucleation rate from the new approximate prefactor-exponent form: top to bottom, J hetero= 100, 1, and 0.01 cm−3s−1 for N=1 cm−3. Markers: results from the double-summation calculation for mean first passage time, J 1=1 cm−3s−1. These show excellent agreement with the approximate result (middle curve).
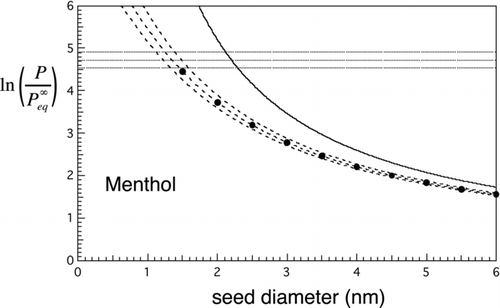
FIG 5 Nucleation rates for water. Solid curve is the Kelvin limit. Dashed lines and curves are contours of constant nucleation rate. Horizontal lines: contours of constant homogeneous nucleation rate: top to bottom, J homo=100, 1, and 0.01 cm−3s−1. Dashed curves give similar contours for the heterogeneous nucleation rate from the new approximate prefactor-exponent form: top to bottom, J hetero= 100, 1, and 0.01 cm−3s−1 for N=1 cm−3. Markers: results from the double-summation calculation for mean first passage time, J 1= 1 s−1. These show excellent agreement with the approximate result (middle curve).
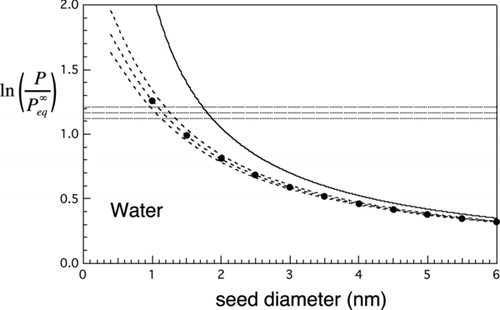
TABLE 1 Parameters and scaling properties for the four working fluids included in and the theoretical minimum particle size (d min seed) that can be detected by each for N=1 cm−3 at the threshold conditions J homo=J hetero=1. Other properties include the critical saturation ratio for homogeneous nucleation, S cr; dimensionless corresponding states parameter, Ω/T; log vapor saturation ratio in unstable equilibrium with the minimum detectable particle size, h; molecular number concentration of vapor in equilibrium with bulk liquid, n eq v ; and the volume per molecule of bulk liquid working fluid, v 1. Note comparative constancies of homogeneous nucleation barrier height and f min=n min seed/g*. Data sources: l-menthol, Becker and Reiss (Citation1978); n-nonane, Rudek et al. (Citation1996); n-butanol, Magnusson et al. (Citation2003); water: Wölk and Strey (Citation2001)
FIG 6 Curves of equal heterogeneous and homogeneous nucleation rates (SNR=1) for n-butanol. Logarithm of ![]()
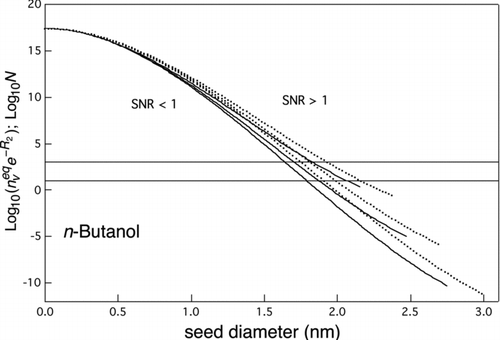
FIG 7 Scaled nucleation rate. Dashed hyperbolic curves: contours of constant homogeneous nucleation barrier height, 50 kT (lower curve) and 70 kT (upper curve). The region between these curves provides a good indication of homogeneous nucleation threshold range for most substances. Markers show four candidate working fluids and are centered on the critical cluster size and critical saturation ratio (as indicated here for the case of nonane), which for each fluid gives J homo=1 cm−3s−1. Error bars show a four order of magnitude range in nucleation rate from J homo=0.01 cm−3s−1 to J homo=100 cm−3s−1. No error bar means that the height of the symbol itself exceeds this range. The solid curve is the Kelvin curve for nonane (Ω/T=2.40 at T=300 K). The horizontal and vertical dotted lines for nonane mark the logarithm of its critical saturation ratio ln(S cr) and g*, respectively. The area of the rectangle bounded by these lines and the axes is twice the reduced barrier height, W * homo/kT. The caret marks the N=1 cm−3 detection limit for nonane.
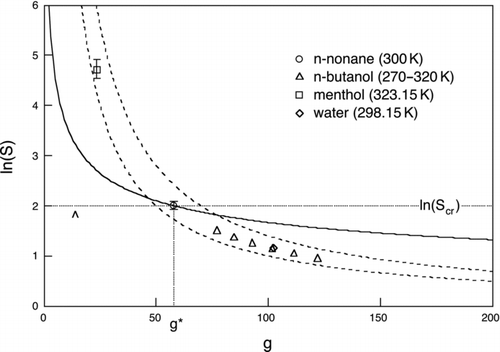
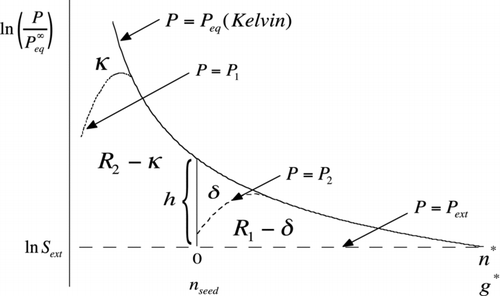
FIG 8 Area construction similar to but illustrating the effect of interactions that lower the equilibrium vapor pressure relative to the Kelvin curve (solid curve). The dotted curve, which only departs from Kelvin at the smallest cluster sizes, results in a lowering of the barrier height for homogeneous nucleation to R 1+R 2−κ. The dashed curve shows lowering of the heterogeneous barrier from R 1 to R 1−δ. Here, h is the length of the vertical line segment (Equation (S18)). Note that the abscissa (upper scale) has been shifted in the heterogeneous case to tally only the number of molecules of condensed working fluid. The lower scale, which runs out to g*, applies to the homogeneous case.