Figures & data
FIG.1 Schematic of the flame configurations to synthesize IONPs. In the DF configuration, (a), the fuel is injected into the center annulus with air on the outside. In the IDF configuration, (b), the oxidizer is injected into the center annulus with fuel injected in the outside concentric flow. (Color figure available online.)
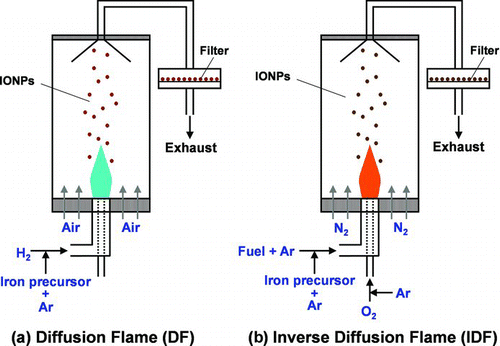
TABLE 1 Flow conditions used to synthesize IONPs
TABLE 2 Summary of particle properties
FIG.2 Transmission electron microscope (TEM) images of the flame-synthesized IONPs (Flames A–E correspond to the IONPs synthesized in the IDF configuration, and maghemite, γ-Fe2O3 corresponds to the IONPs synthesized in the DF configuration) and a goethite-based commercial sorbent (E33). For all samples, the particles form large aggregates composed of smaller primary particles.
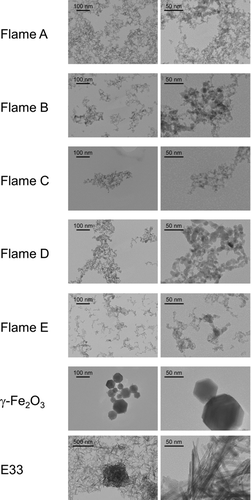
FIG.3 Powder X-ray diffraction patterns of flame synthesized iron oxide samples. Vertical ticks represent main diffraction peaks for maghemite.
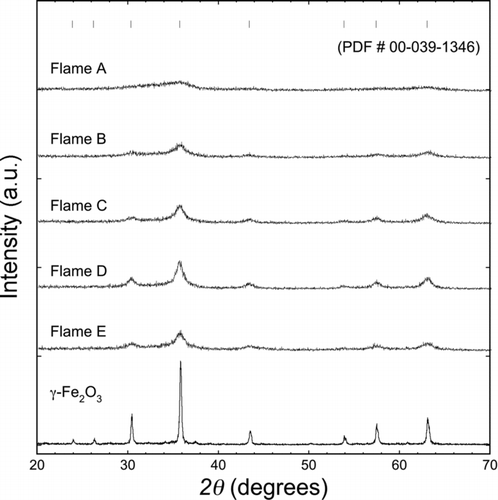
FIG.4 (a) As(V) adsorption isotherms of the flame-synthesized IONPs at pH 7. As(V) adsorption isotherms of Fe3O4 nanocrystals (Yavuz et al. Citation2006) and a goethite-based common adsorbent, E33 (Kanematsu et al. Citation2011) are included for reference. (b) Surface area normalized As(V) adsorption isotherms of the flame synthesized IONPs at pH 7. (Color figure available online.)
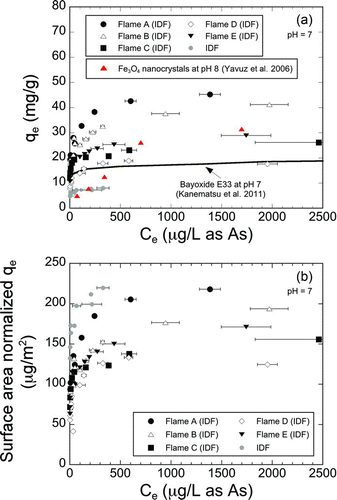
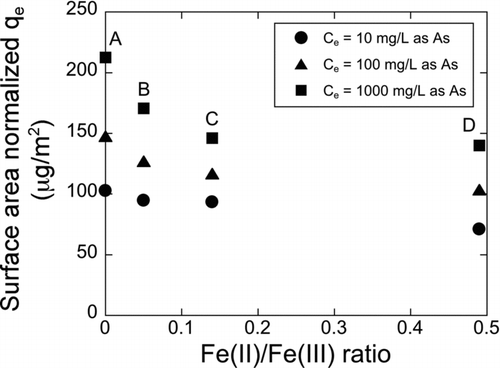
FIG.5 Influence of oxidation state, indicated by Fe(II)/Fe(III) ratio, on the surface area normalized As(V) adsorption capacity of the IONPs (Flames A to D) at three different liquid phase equilibrium As(V) concentrations.