Figures & data
TABLE 1 Properties of the dicarboxylic acids modeled in this study
FIG. 1 Comparison of temperatures and vapor concentrations predicted by the 1-D plug-flow and the 2-D laminar-flow models. Tb and cb are the bulk temperature and vapor concentration obtained by the 1-D model. Tavg and cavg are the cup-average temperature and vapor concentration obtained by the 2-D model. Tw is wall temperature. csw and csb are the saturation vapor concentrations at Tw and Tb . (Color figure available online.)
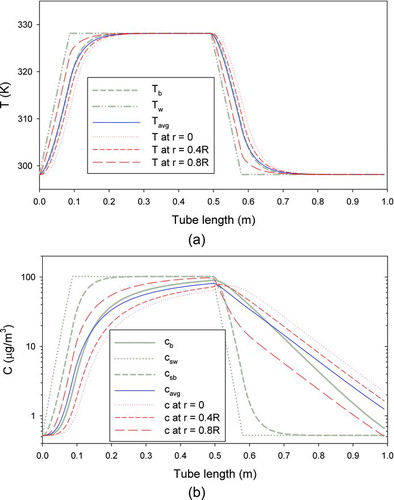
FIG. 2 Comparison of the measured and model-predicted MFR: (a) model differences, for butanedioic acid; (b) effect of initial aerosol concentration (COA), for hexanedioic acid. (Color figure available online.)
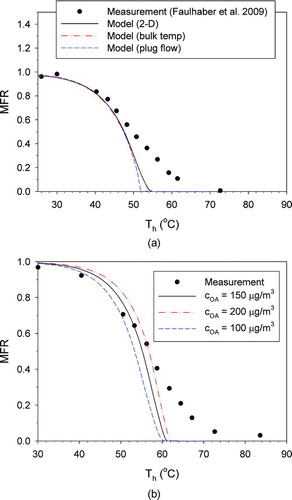
FIG. 3 Sensitivity test results for hexanedioic acid for (a) evaporation coefficient; (b) saturation vapor pressure at 25°C; and (c) enthalpy of vaporization. (Color figure available online.)
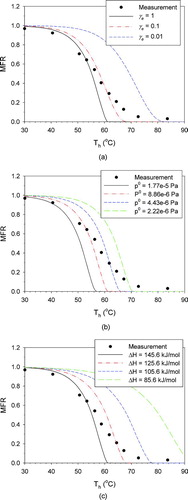
TABLE 2 Summary of the values for the saturation vapor pressure at 25°C (p0) and the enthalpy of vaporization (ΔHv ) reported by different investigators
FIG. 4 Adjustment of the evaporation coefficient (γe) to fit the measured MFRs: (a) butanedioic acid; (b) hexanedioic acid. Also shown is the influence of variability/uncertainty in the initial aerosol concentration (COA) for the central values of γe. (Color figure available online.)
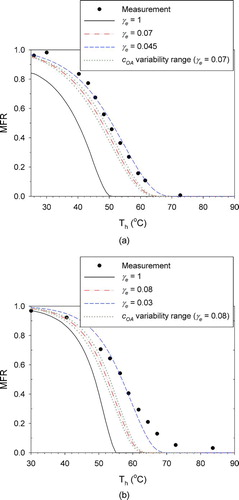
FIG. 5 Adjustment of the reference saturation pressure and the enthalpy of vaporization to fit the measured MFRs: (a) butanedioic acid; (b) hexanedioic acid. (Color figure available online.)
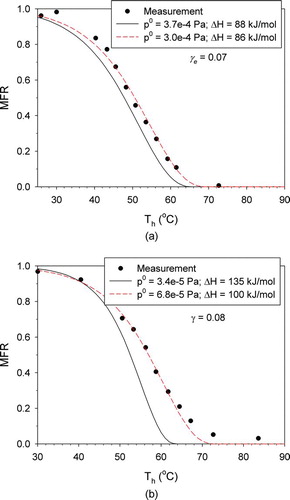
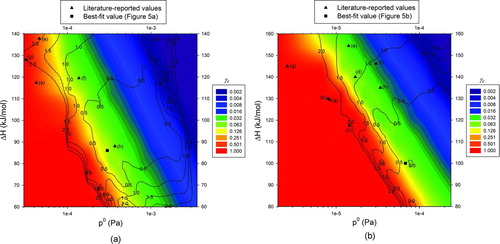
FIG. 6 Contour plots for evaporation coefficient (colored) and deviation percent (contours) as functions of the reference saturation vapor pressure and the enthalpy of vaporization: (a) butanedioic acid; (b) hexanedioic acid. Literature-reported values listed in and the best-fit values shown in Figure 5 are also shown.