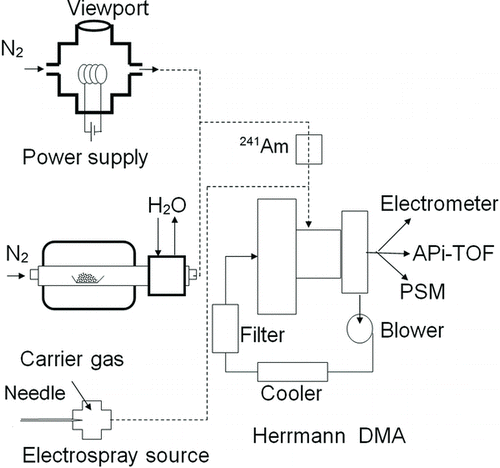
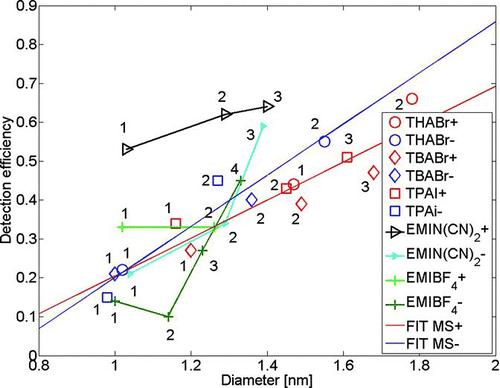
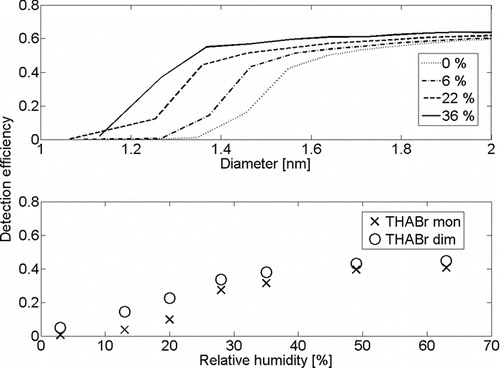
Figures & data
FIG. 2 The average mass spectra of negative ammonium sulfate, sodium chloride, and tungsten oxide with the overlaid electrometer signal. The chemical composition of the clusters is given in the text.
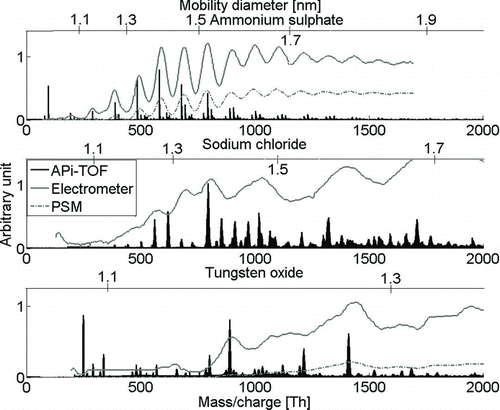
FIG. 3 Mobility diameter versus mass of negatively charged ammonium sulfate. Bands of doubly and triply charged clusters are observed. (Color figure available online.)
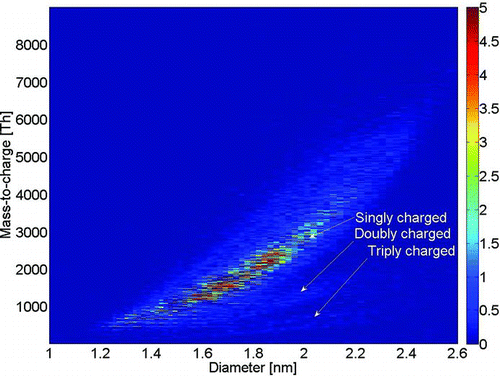
FIG. 4 Mass spectra of ammonium sulfate, sodium chloride, tungsten oxide, and silver with a mobility diameter of 1.26 nm in negative polarity. Fragmentation and different apparent densities of clusters are observed.
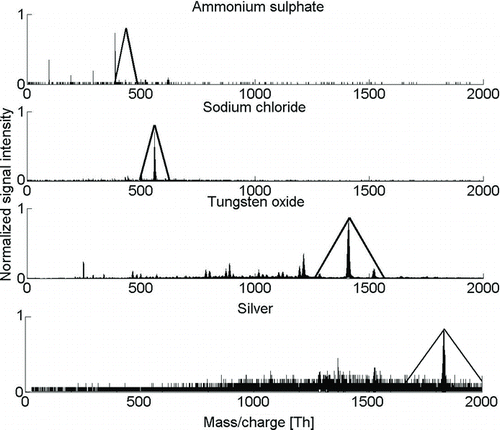
FIG. 5 Fraction of the known clusters’ mass spectrometer signal to the total signal at the selected size as a function of mobility diameter. Generation of clean samples proved to be difficult.
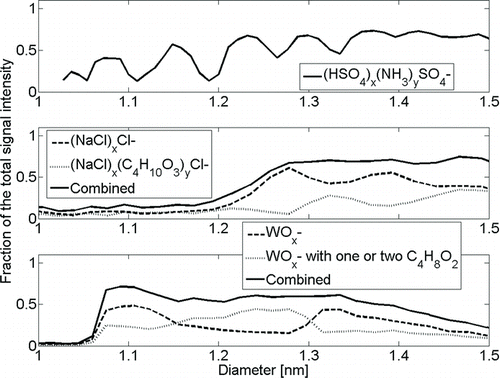
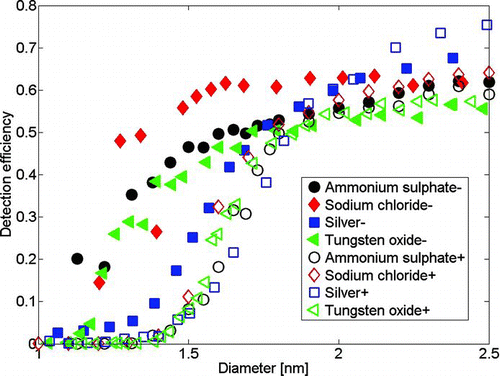
FIG. 6 The detection efficiency curves of the PSM for various ions. The positively charged ions are organics below 1.5 nm and they activate worse than inorganic samples used here. (Color figure available online.)