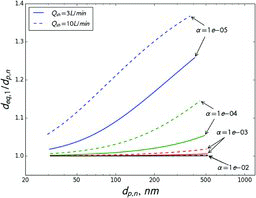
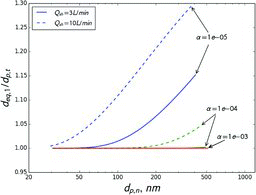
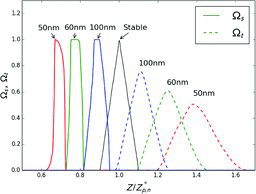
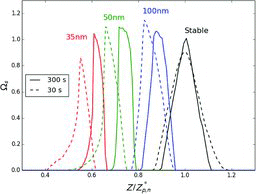
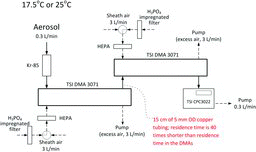
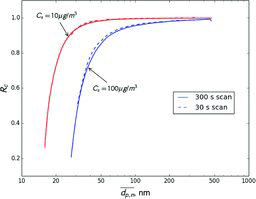
Figures & data
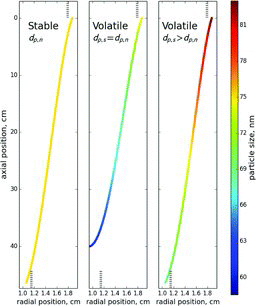
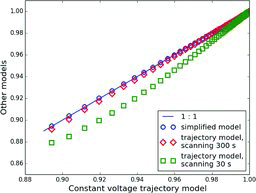
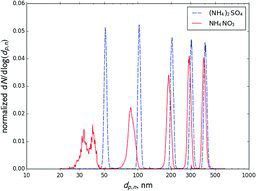
Table 1 Summary of measured midpoint sizes of ammonium sulfate (AS) and ammonium nitrate (AN) aerosol during different experiments. All sizes are in nm
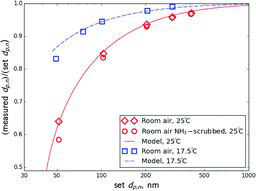
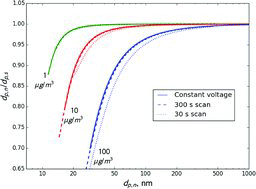
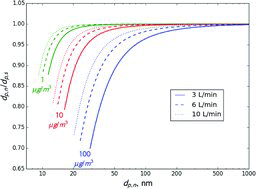
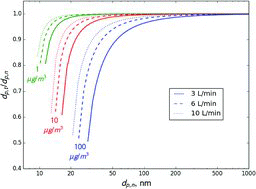
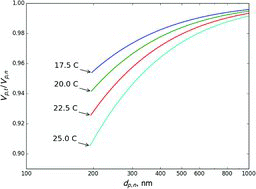
Table 2 Estimated ratio of the transmitted to set mass when selecting 300 nm ammonium nitrate aerosol using a single DMA