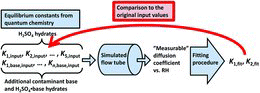
Figures & data
Table 1 Clusters included in the systems studied using the B3LYP/CBSB7//RICC2/aug-cc-pV(T+d)Z cluster energies. The number of water molecules in the cluster is indicated in parenthesis. Additional clusters, that were included only in the simulations where the system was extended to clusters containing up to two H2SO4 and two base molecules, are marked with an asterisk; otherwise only clusters containing up to one H2SO4 and one base molecule were included
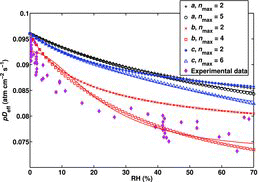
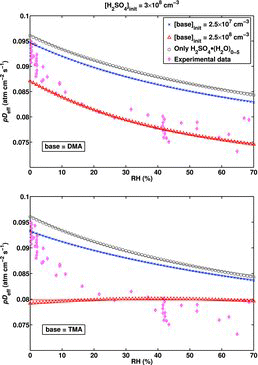
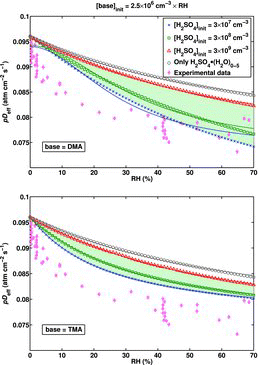
![Figure 5 Effective diffusion coefficient as a function of RH for the H2SO4–DMA system extended to include larger clusters, calculated using the B3LYP/CBSB7//RICC2/aug-cc-pV(T+d)Z Gibbs free energies. The shaded (green) area presents the spread of the results for pDeff as the initial H2SO4 concentration varies between 3 × 107 cm−3 and 3 × 109 cm−3. The upper limit of the shaded area corresponds to [H2SO4]init = 4 × 108 cm−3.](/cms/asset/9638d4be-d95e-4484-8d18-de2cc4e8d65d/uast_a_903556_f0005_oc.jpg)