Figures & data
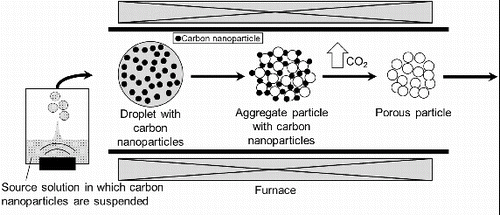
FIG. 2. Apparatus for synthesizing SOFC electrolyte material particles using ultrasonic spray pyrolysis (CNA-USP).
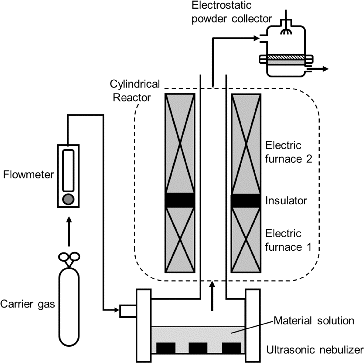
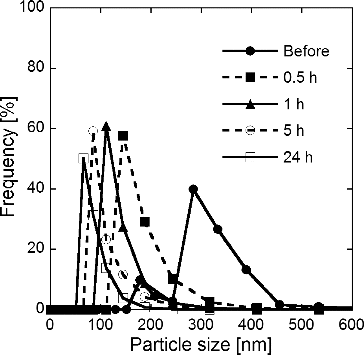
TABLE 1 Specific surface areas Sw obtained from N2 adsorption isotherms of Gd0.1Ce0.9O1.95 particles before and after 24-h grinding; particles were synthesized at TH = 1073 K, Cc = 0, and 16 g L−1
FIG. 3. SEM images of particles synthesized using CNA-USP method from aqueous solutions of Ce(NO3)3 6H2O and Gd(NO3)3 6H2O dissolved in stoichiometric ratios of Gd0.1Ce0.9O1.95 at Cc = (a) 0, (b) 8, (c) 16, and (d) 24 g L−1, at Ctotal = 0.02 mol L−1, TH = 1273 K, and tr = 9.4 s.
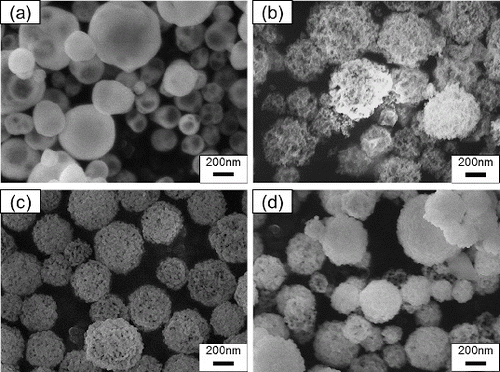
TABLE 2 Geometric mean diameter Dpg and geometric standard deviation σg obtained from SEM images for Gd0.1Ce0.9O1.95 particles shown in Figure 3; particles were synthesized at Cc = 0–24 g L−1 and TH = 1273 K
TABLE 3 Concentrations of Ce3+ and Gd3+, CCe3+ and CGd3+, and atomic ratios of Ce3+ : Gd3+ in precursor solutions to which carbon nanoparticles were added
TABLE 4 Average crystallite size Dcry obtained from XRD patterns of Gd0.1Ce0.9O1.95 particles shown in Figure 4, geometric mean diameter Dpg and geometric standard deviation σg obtained from SEM images shown in Figure 5; particles were synthesized at TH = 873–1473 K and Cc = 16 g L−1
FIG. 4. XRD patterns of particles synthesized using CNA-USP method from aqueous solutions of Ce(NO3)3 6H2O and Gd(NO3)3 6H2O dissolved in stoichiometric ratios of Gd0.1Ce0.9O1.95 at TH = (a) 873 K, (b) 1073 K, (c) 1273 K, and (d) 1473 K compared to (e) JCPDS data for Gd0.1Ce0.9O1.95, at Ctotal = 0.02 mol L−1, Cc = 16 g L−1, and tr = 9.4 s.
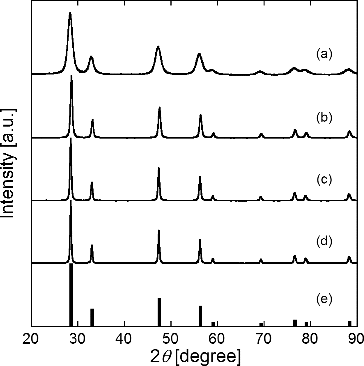
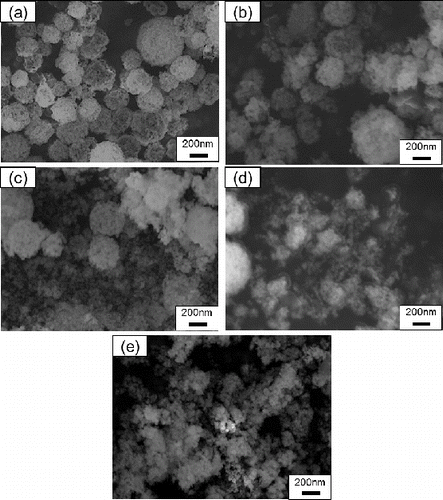
FIG. 5. SEM images of Gd0.1Ce0.9O1.95 particles synthesized using CNA-USP method at TH = (a) 873 K, (b) 1073 K, (c) 1273 K, and (d) 1473 K, at Ctotal = 0.02 mol L−1, Cc = 16 g L−1, and tr = 9.4 s.

FIG. 6. TG data of (a) Gd0.1Ce0.9O1.95 particles synthesized using CNA-USP method at TH = 873 K, 1073 K, 1273 K, and 1473 K, at Ctotal = 0.02 mol L−1, Cc = 16 g L−1, and tr = 9.4 s and (b) carbon nanoparticles added in the precursor solutions.
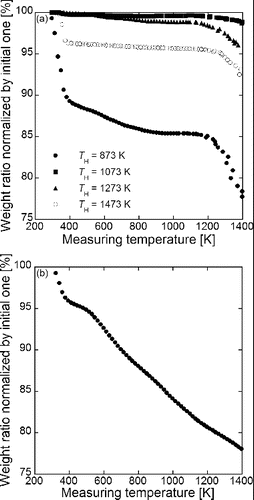
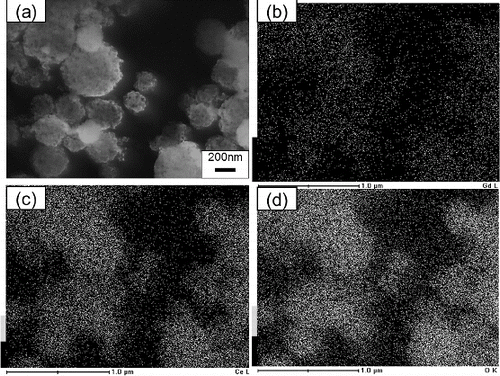
FIG. 7. (a) SEM images of Gd0.1Ce0.9O1.95 particles synthesized using CNA-USP method at Cc = 16 g L−1 and EDX images of (b) Gd, (c) Ce, and (d) O contained in them, at Ctotal = 0.02 mol L−1, TH = 1073 K, and tr = 9.4 s.